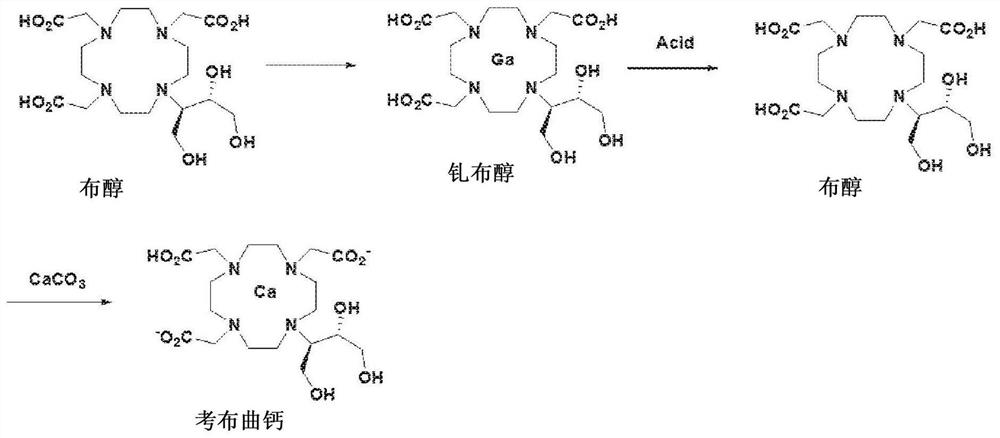
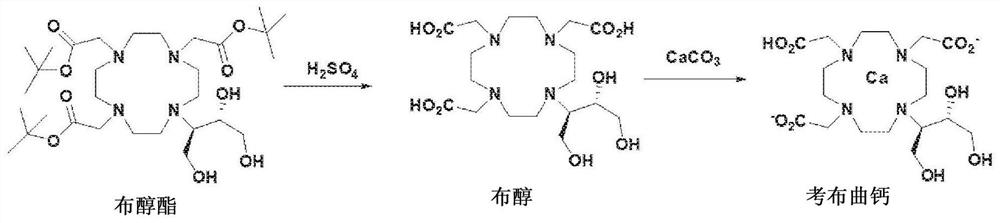
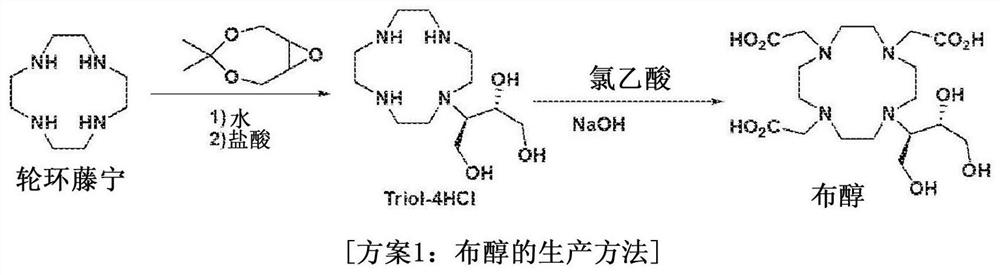
Method for preparing calcobutrol
A production method and the technology of butol, which are applied in the field of producing high-purity calcobutrol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Crude Butanol (2,2',2"-(10-((2R,3S)-1,3,4-trihydroxybutan-2-yl)-1,4,7,10) - Production of tetraazacyclododecane-1,4,7-triyl)triacetic acid)
[0050] Dissolve 100 grams of rotenin (1,4,7,10-tetraazacyclododecane) in 500 milliliters of purified water and heat to a temperature of 40-45° C., slowly drop 84 grams of 4,4-Dimethyl-3,5,8-trioxabicyclo[5,1,0]octane. After reacting for 24 hours, 408 ml of hydrochloric acid was added dropwise to the reaction solution, followed by stirring at 75°C for 2 hours. The reaction solution was concentrated in vacuo, and 500 ml of ethanol was added thereto. The resulting solution was stirred and cooled at reflux, and the crystals formed were filtered.
[0051] The formed crystals were dissolved in 500 ml of purified water, and 170 g of chloroacetic acid was added thereto. The pH of the resulting solution was adjusted to 9-10 using 45% sodium hydroxide (NaOH), heated to 70°C, and stirred for 12 hours while maintaining the pH...
Embodiment 2
[0055] Example 2: Butanol (2,2',2"-(10-((2R,3S)-1,3,4-trihydroxybutan-2-yl)-1,4,7,10-tetraazole Purification of heterocyclododecane-1,4,7-triyl)triacetic acid)
[0056] Concentrated butanol was dissolved in 500 ml of purified water and passed through a column containing a mixture of 100 ml of anion (IRA-67) and 100 ml of cation (IR-120). The passing butanol eluate was recycled to the column containing the mixture, and this operation was repeated several times. Wash the resin, combine the eluent and washing solution, adjust the pH value to 3.5-4.5 with cations, filter, and then concentrate. 100 ml of purified water was added to the concentrated solution, and after it was completely dissolved, it was heated to 80° C., and then 500 ml of methanol was added thereto. The resulting solution was cooled slowly to room temperature, and a small amount of seed crystals were added thereto. After stirring for 2 days, the crystals formed were filtered. The crystals were vacuum-dried ...
Embodiment 3
[0062] Embodiment 3: the production of calbutril calcium
[0063] 10 g of high-purity butanol (purity: 99.5% or higher) produced in Example 2 was dissolved in 100 ml of purified water, and 2.22 g of calcium carbonate (CaCO 3 ). The mixture was stirred at room temperature for 1 hour, filtered through a 0.2 micron filter, concentrated, and then freeze-dried to obtain 10.7 g of Calcobutrol (yield: 99%, purity: 99.5% or higher).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com