Methods for characterizing disulfide bonds
A technology of disulphide bonds and misconnection of disulfide bonds, which can be used in the preparation of test samples, labels and instruments used in chemical analysis, etc., can solve time-consuming and labor-intensive problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
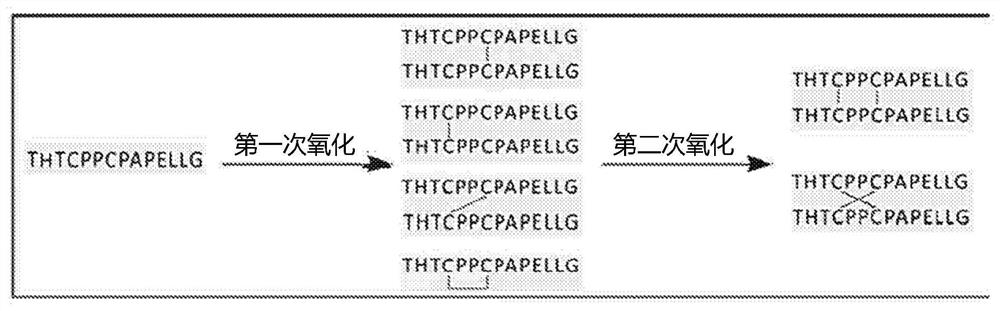
[0072] Example 1: Synthesis of Parallel and Crossed Hinge Peptides
[0073] method :
[0074] cross-linking
[0075] Purchase cysteine-containing peptides from commercial suppliers. By reacting with 1 mM Cu as an oxidizing agent in the presence of air 2+ Incubation results in cross-linking of the peptides. Peptide and Cu 2+ The molar ratio is 5:1.
[0076] N-terminal analysis / Edman degradation
[0077] The cross-linked peptides were suspended in water and placed in a protein sequencer. Peptides were exposed to phenylisothiocyanate (PITC). PITC is coupled to the N-terminal residue to form a PTC polypeptide. Upon addition of trifluoroacetic acid to the reaction, the PTC N-terminal residue undergoes acid cleavage, resulting in the release of the labile ATZ-amino acid. Separate the ATZ-amino acid from the peptide solution into a transformation flask with ethyl acetate. ATZ-amino acids were converted to stable PTH-amino acids with 25% TFA in water (v / v). The PTH-amino ac...
Embodiment 2
[0081] Example 2: Analysis of the hinge regions of two mAbs
[0082] method
[0083] Hinge disulfide bond characterization
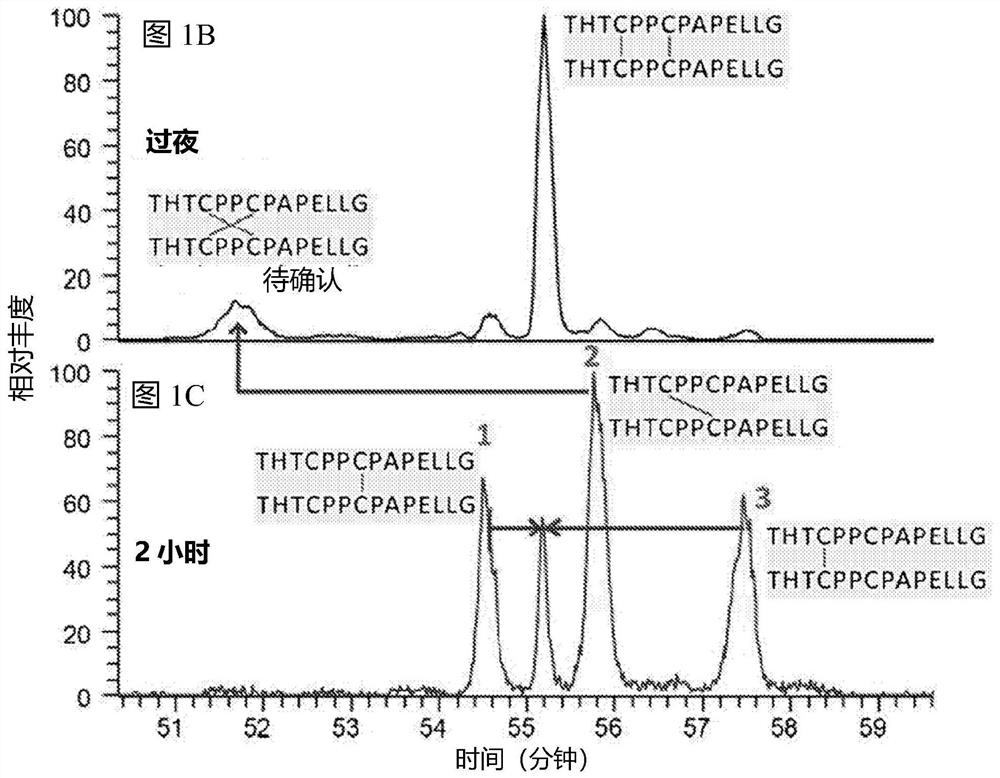
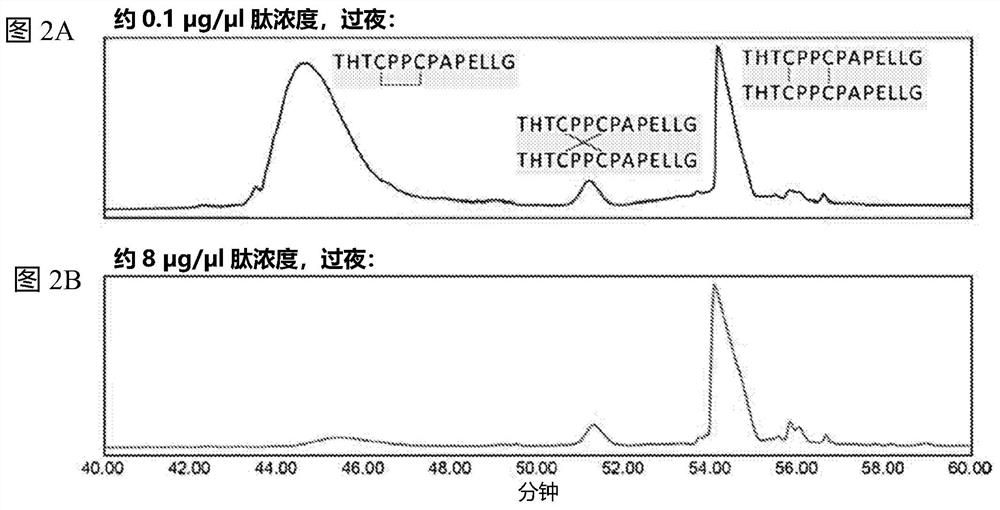
[0084] Antibodies are first digested into peptides. IgGl antibodies were subjected to double enzyme digestion using proteinase K followed by trypsin. IgG4 antibodies were subjected to FabRICATOR followed by trypsin digestion. Hinge peptide standards were prepared as described above using the hinge region sequences of the IgGl and IgG4 antibodies used to prepare the peptides. The digested peptide mixture was subjected to LC-MS analysis. Hinge peptide standards were also subjected to LC-MA analysis. Retention time analysis was performed to compare the retention time of the antibody peptide to that of the hinge peptide standard.
[0085] result
[0086] IgG1 mAb1 was subjected to digestion into peptides, and the resulting peptides were subjected to LC / MS analysis. The hinge peptide standard above was also subjected to LC / MS analysis. Such as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com