Preparation and application of retinoic acid induced protein 16 specific polyclonal antibody
An antibody and antigen protein technology, applied in the field of biomedicine, can solve the problems of not being able to specifically recognize the RAI16 protein, not being able to knock out mouse validation, etc., and achieve the effects of good specificity, high titer, and low preparation cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of anti-RAI16 92-292AA antibody
[0060] Anti-RAI16 92-292AA antibody was prepared as follows:
[0061] (1) Analysis of RAI16 antigenic epitope: using DNAStar software to analyze the amino acid sequence of RAI16 protein, the analysis results are as follows figure 1 As shown, the 92-292 amino acids of RAI16 protein have excellent hydrophilic structure, flexible region, antigenic index and surface probability structure. Determine the 201 amino acids at positions 92-292 of the RAI16 protein as the expressed immunogen protein sequence:
[0062] Its amino acid sequence is as follows:
[0063] lctlgkaeyppgmrqqvfqffskvlsqvqhpllhylsvhrpvqkllrlggtvpgsltekeevqftsvlcskiqqdpellayilegkkiigkkktarestappkdiagyrdkdcphsdalnrdpgldkehcgvpalsihlpaetegpengpgesnlitsllglckskksrlalkaqenilltqvastvast
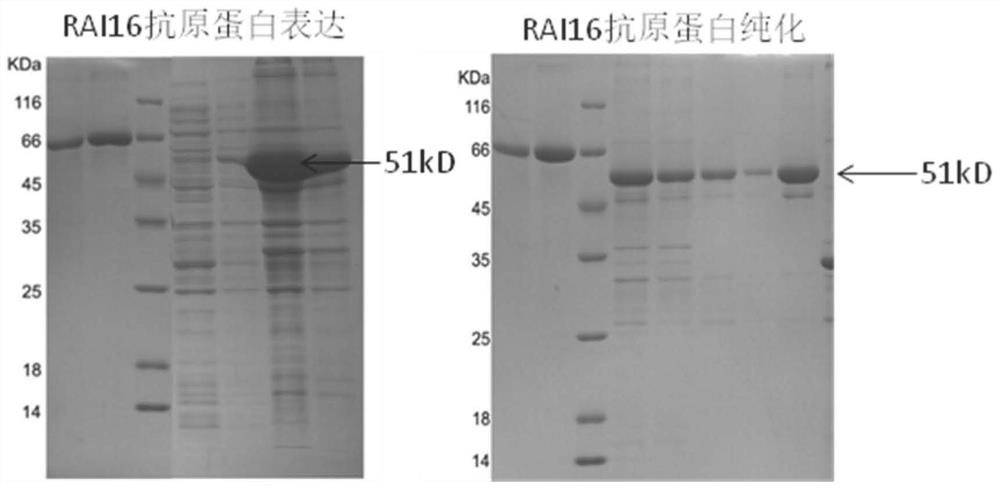
[0064] (2) RAI16 92-292AA protein expression: Construct 92-292AA expression plasmid, use Escherichia coli to express, use affinity chromatography column and HPLC column t...
Embodiment 2
[0067] Example 2: Determination of Anti-RAI16 92-292AA Protein Antibody Titer
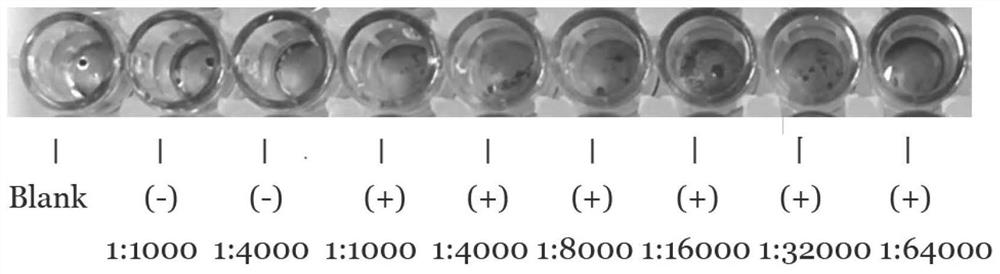
[0068] In this embodiment, ELISA is used to detect the titer of the anti-RAI16 92-292AA protein antibody: the ELISA detection plate is coated with RAI16-BSA at a concentration of 1mg / L, 100ul per well, incubated at 4°C for 8-12h, and then each well is Add 200ul of bovine serum with a concentration of 100ul, block at 37°C for 2h, add double-diluted anti-RAI16 92-292AA protein antibody after washing (normal rabbit serum is added to the control group), incubate at 37°C for 60min, 3 times After washing, add 100 ul of HRP-labeled goat anti-rabbit IgG diluted 1:5000 to each well, incubate at 37°C for 30 min, wash 3 times and develop TMB color, measure the absorbance value of the sample at A450 with a microplate reader, and calculate the antibody efficacy Valence, the antibody titer of this embodiment is 1:64000 (such as image 3 shown).
Embodiment 3
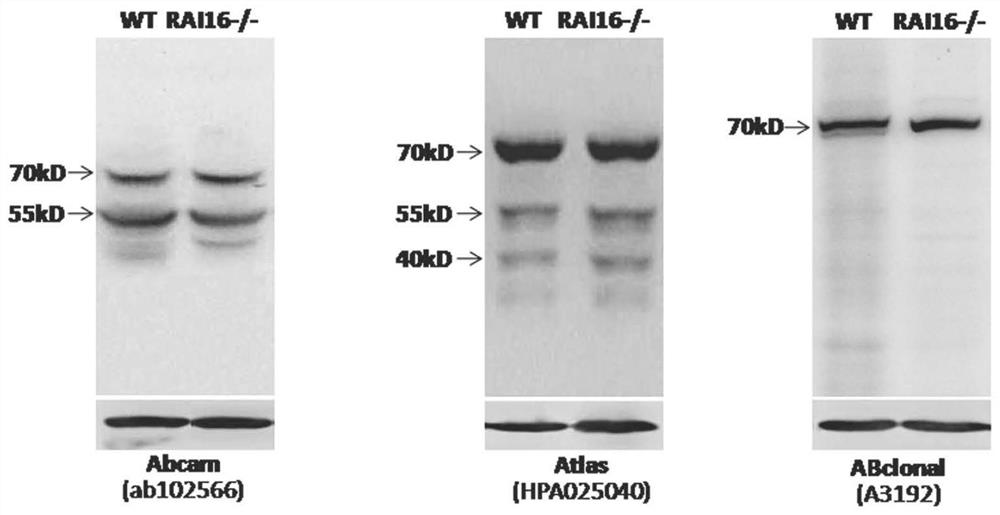
[0069] Example 3: Anti-RAI16 92-292AA protein antibody is used for the detection of RAI16 protein expression
[0070] Western blotting was used to detect the expression of RAI16 protein.
[0071] 1×10 7 Cells or 100mg of mouse colon tissue were added to the lysate (50mM Tris-HCl, 150mM NaCl, 0.5mM EDTA, protease inhibitor cocktail) and lysed at 4°C for 30min, centrifuged at 4°C and 10000g for 20min, and the supernatant was quantified followed by protein samples. Suspend the prepared cell or colon tissue protein samples in 5xSDS loading buffer, heat at 95°C for 10 minutes, place on ice to cool for 10 minutes before loading the samples; after separation by SDS-PAGE gel, transfer to PVDF membrane by wet transfer method; After blocking with skimmed milk powder (at room temperature for 2 hours), add 1:1000 diluted purified RAI16 antibody overnight at 4°C; wash the membrane 3 times with PBST, then add 1:2000 HRP-labeled goat anti-rabbit IgG and incubate at room temperature for 1 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com