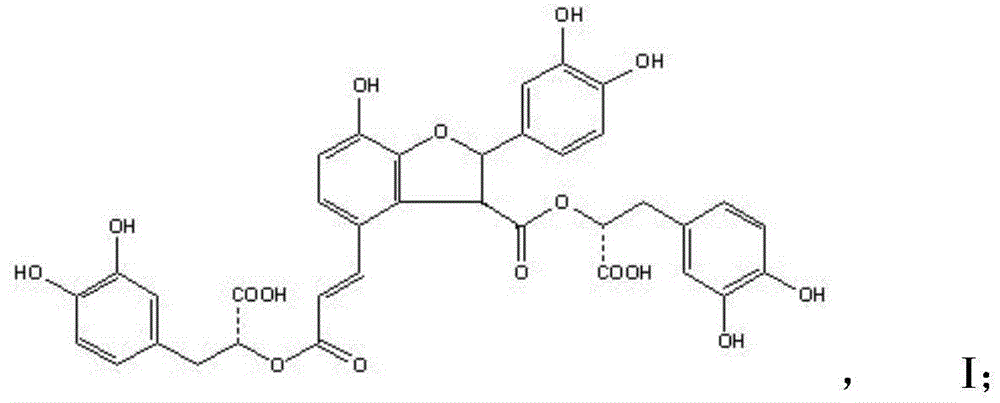
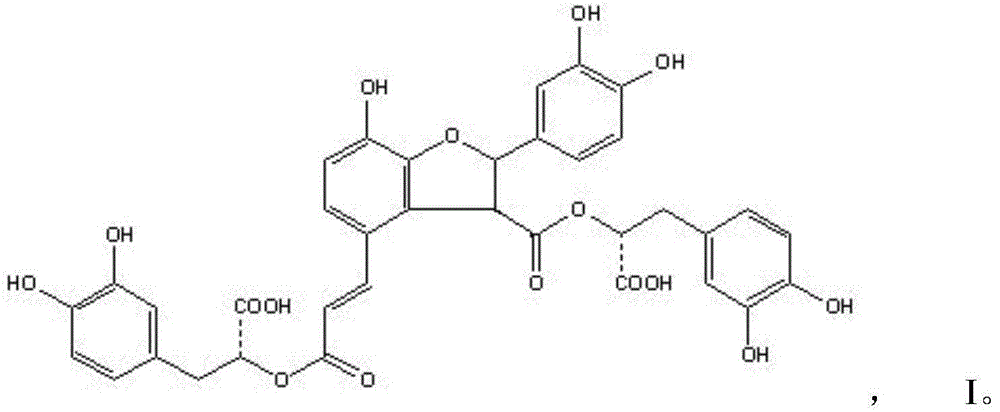
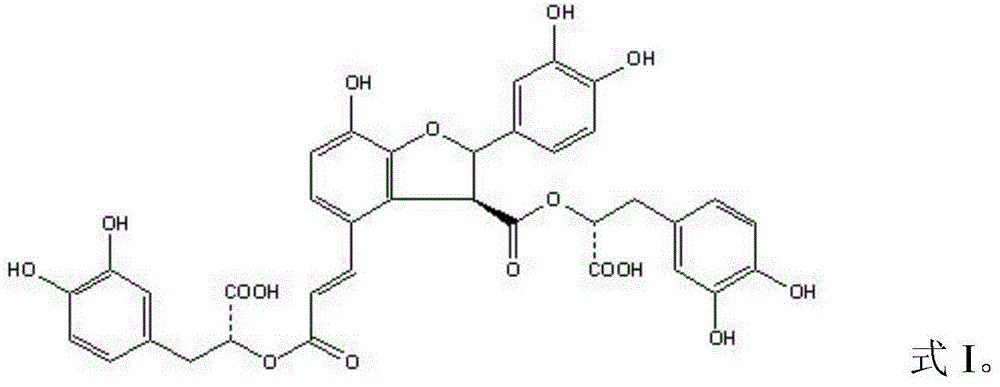
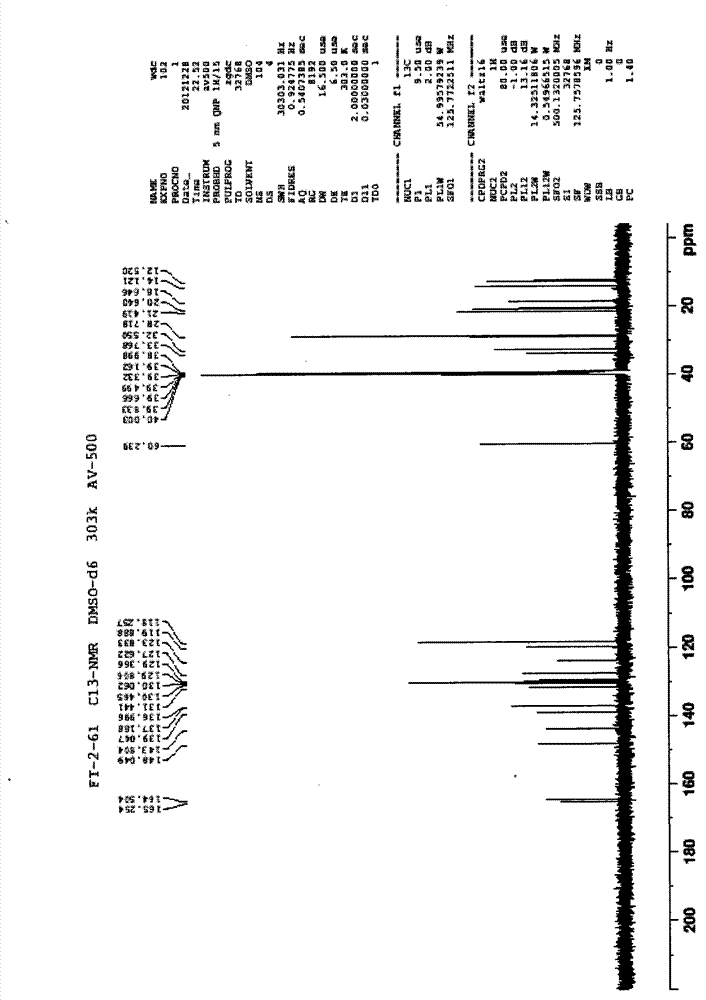
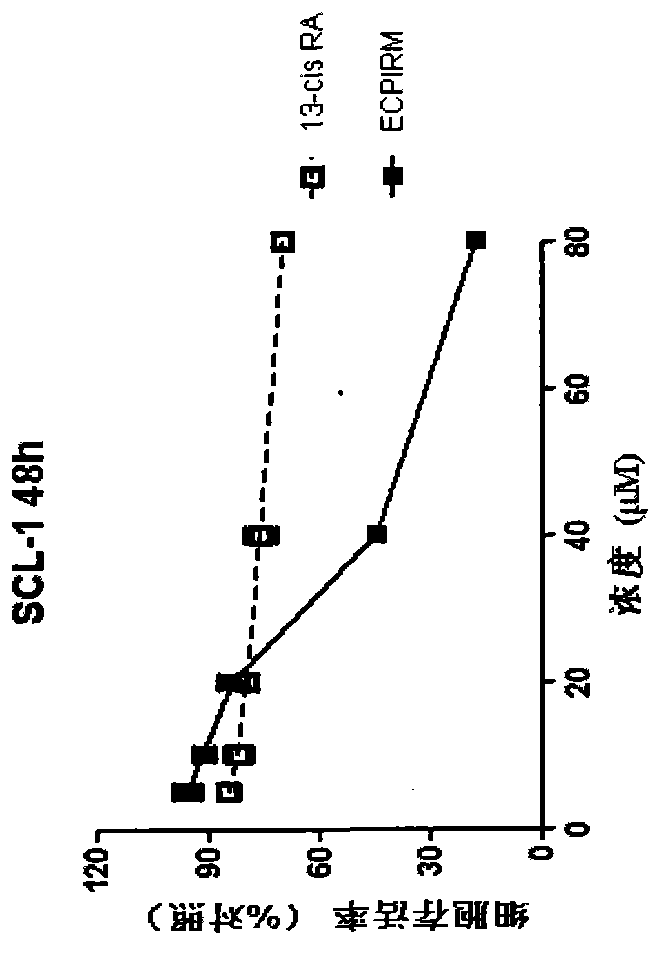
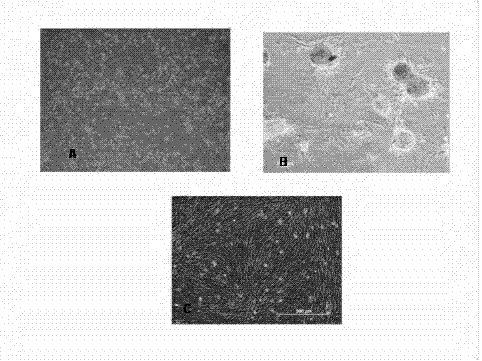
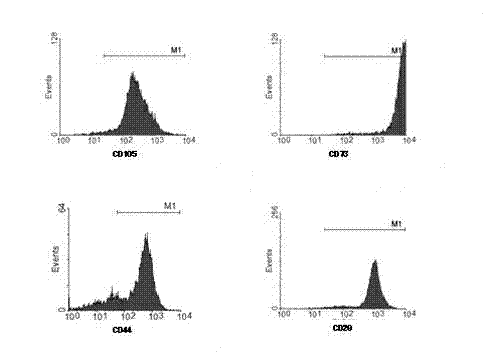
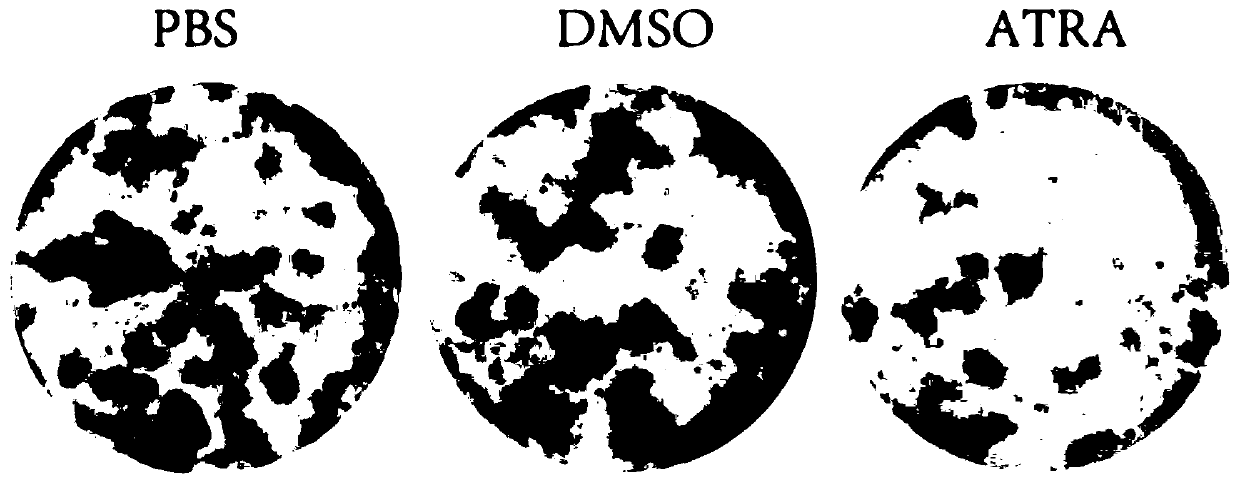
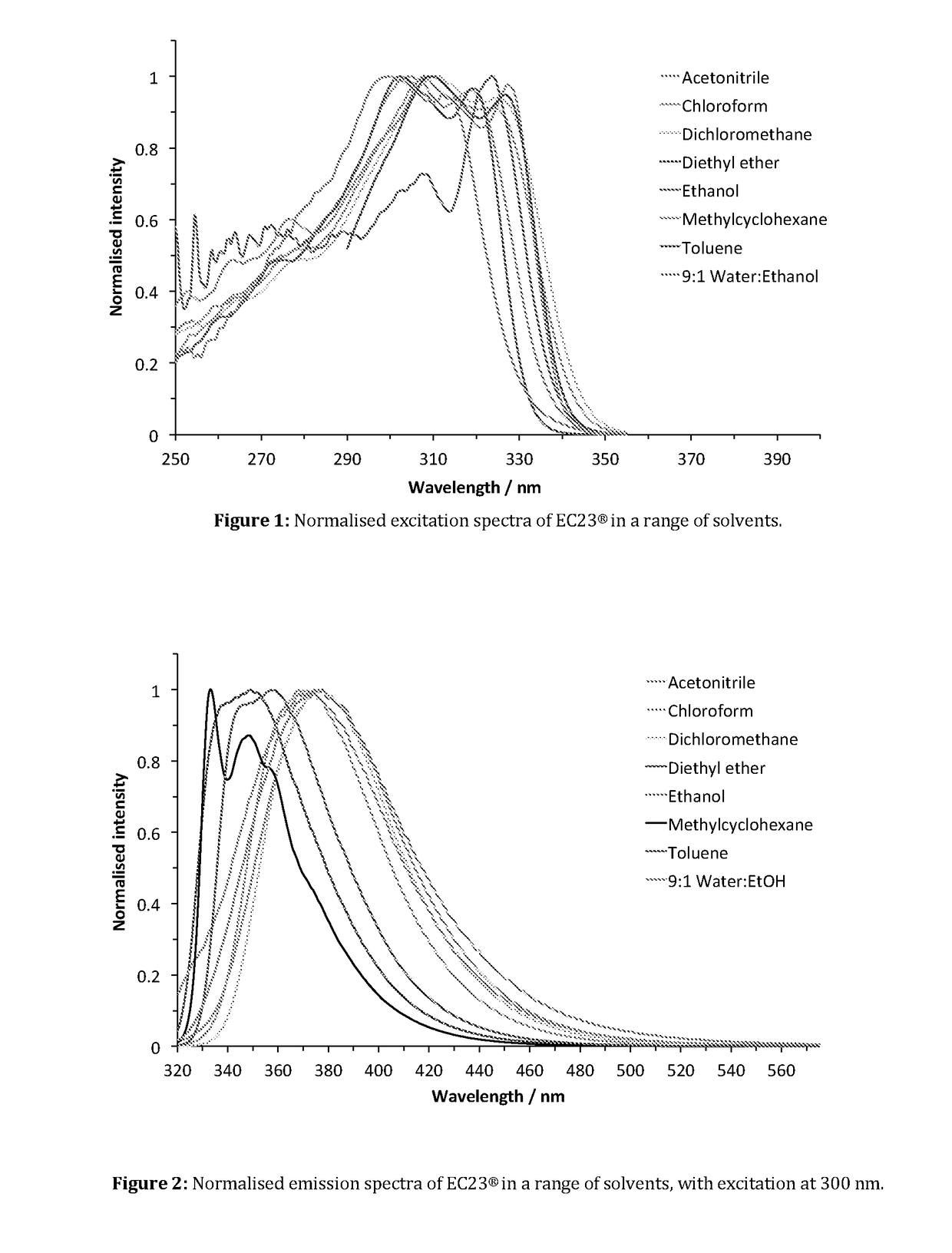
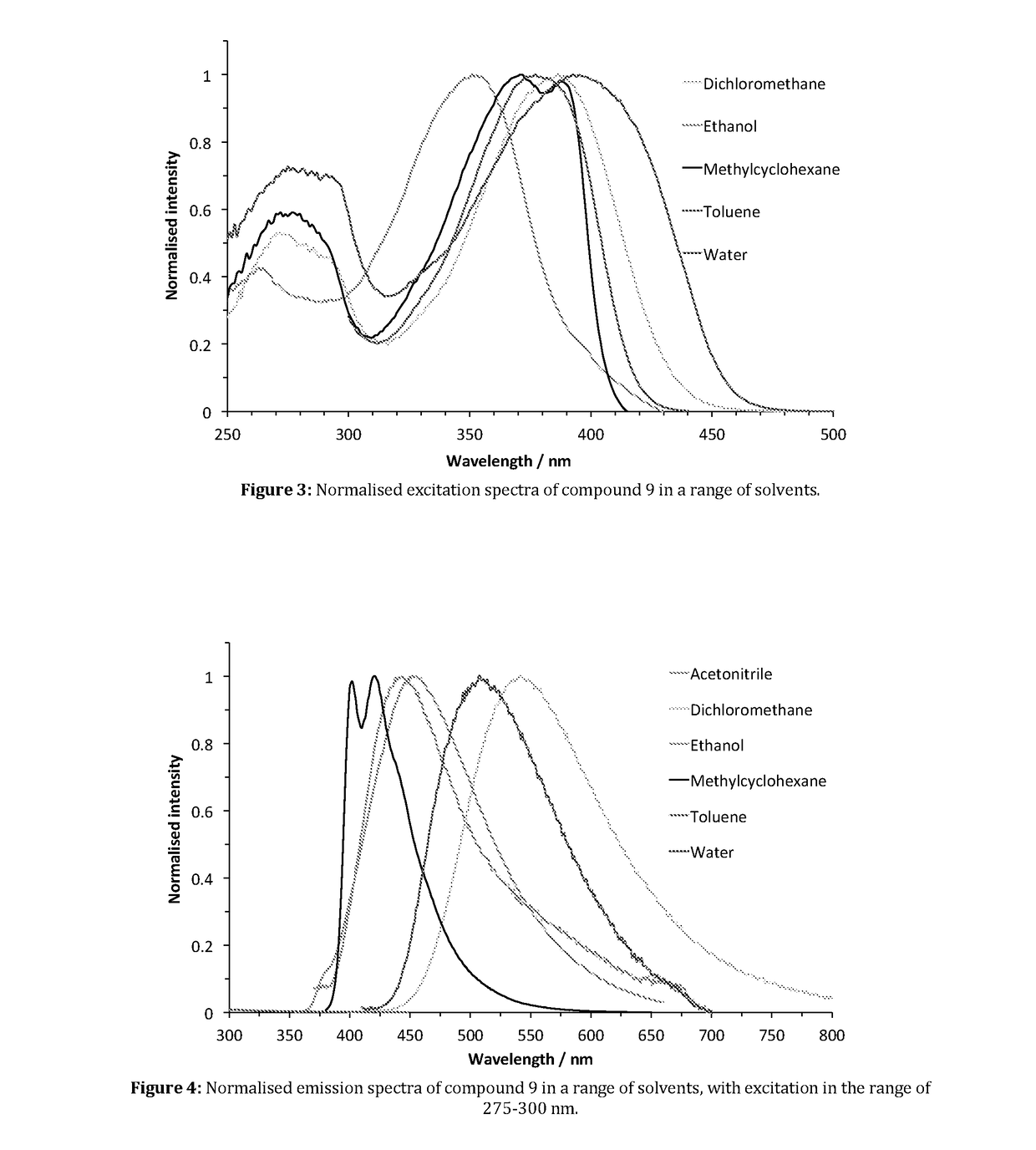
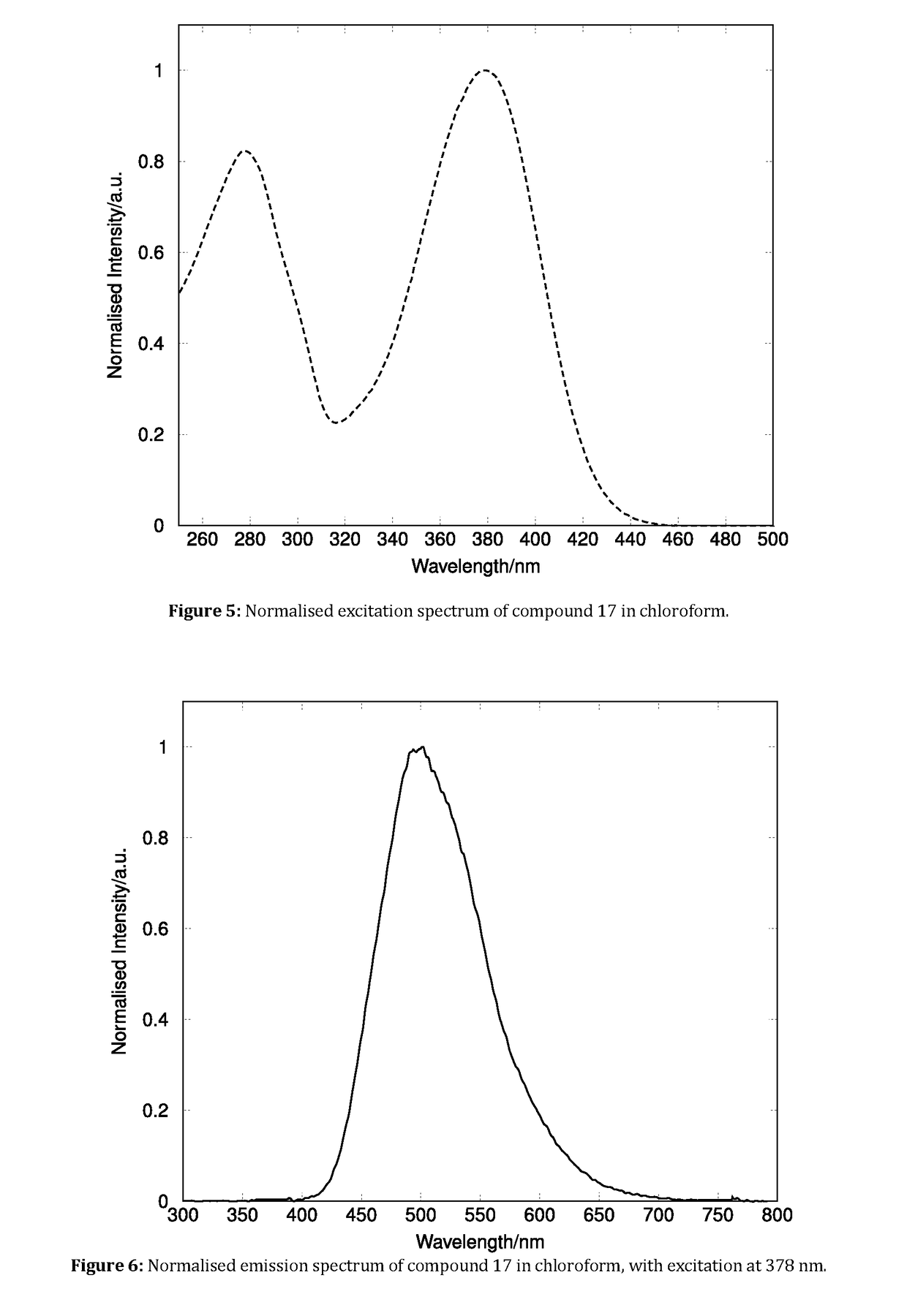
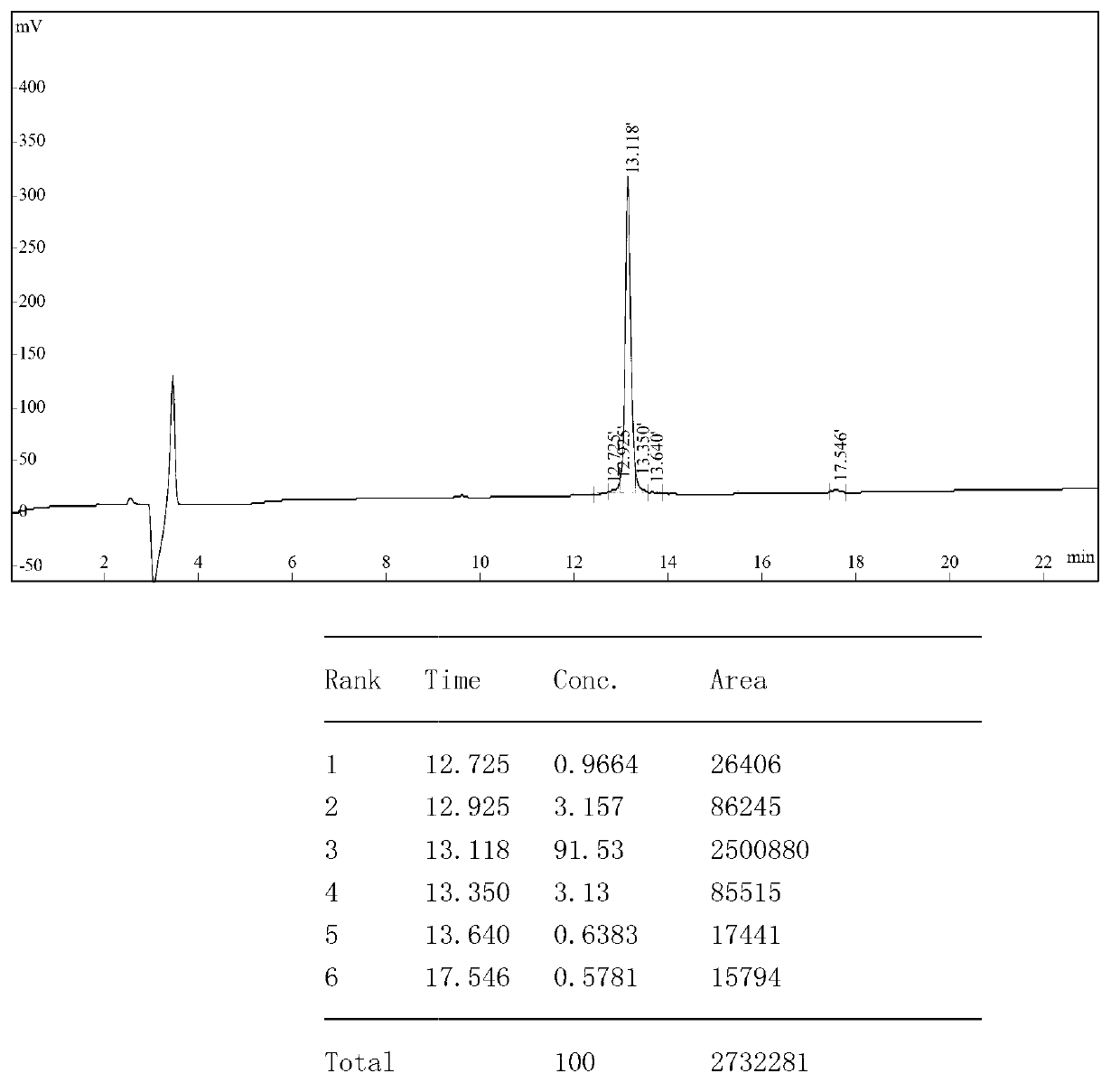
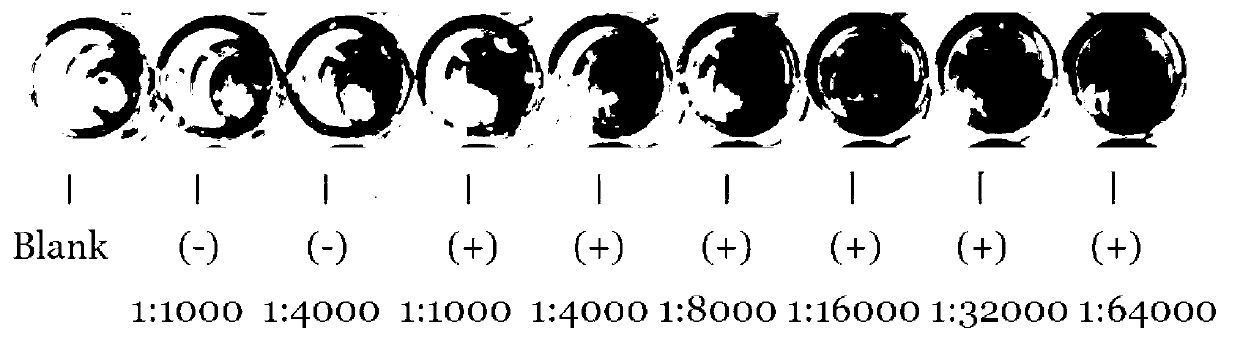
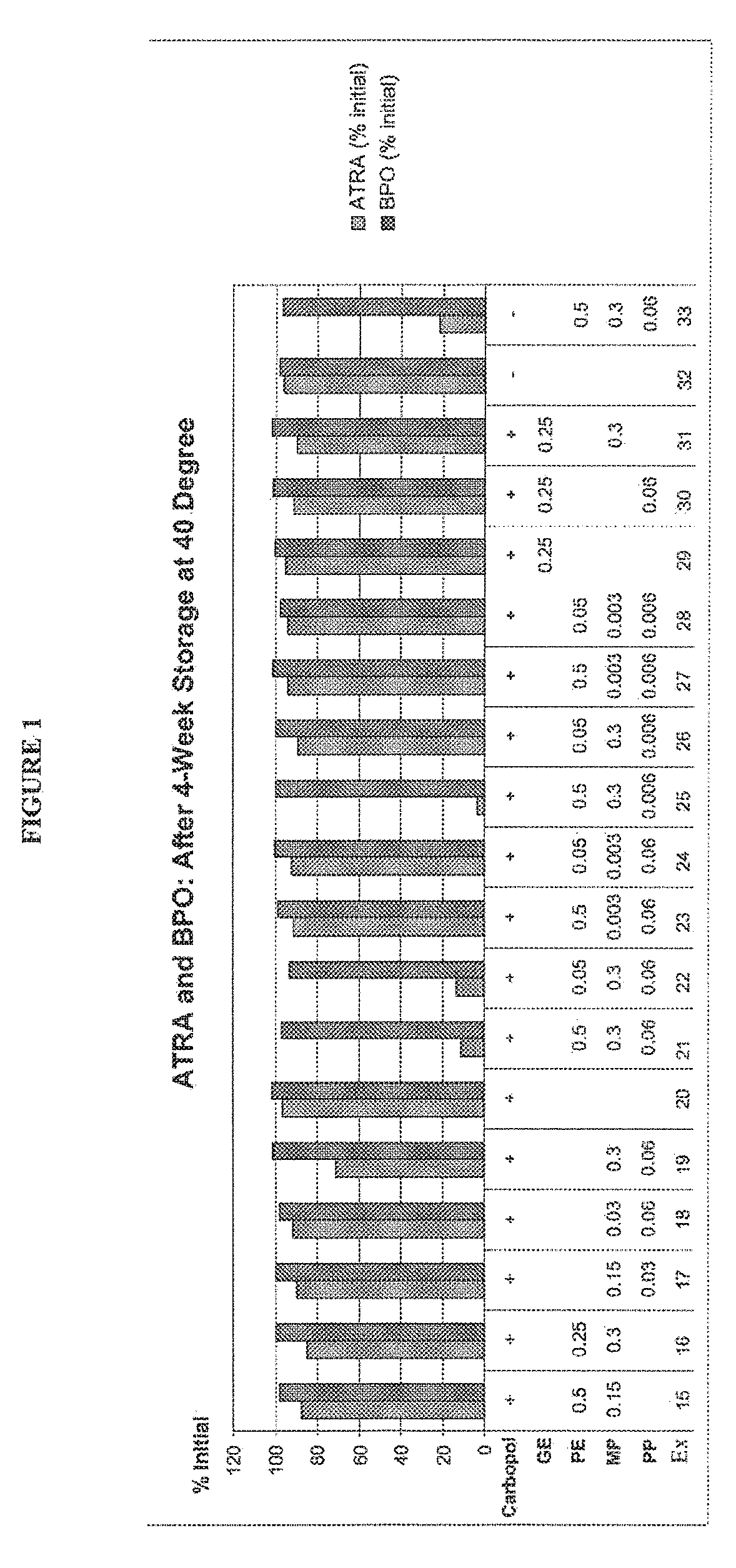
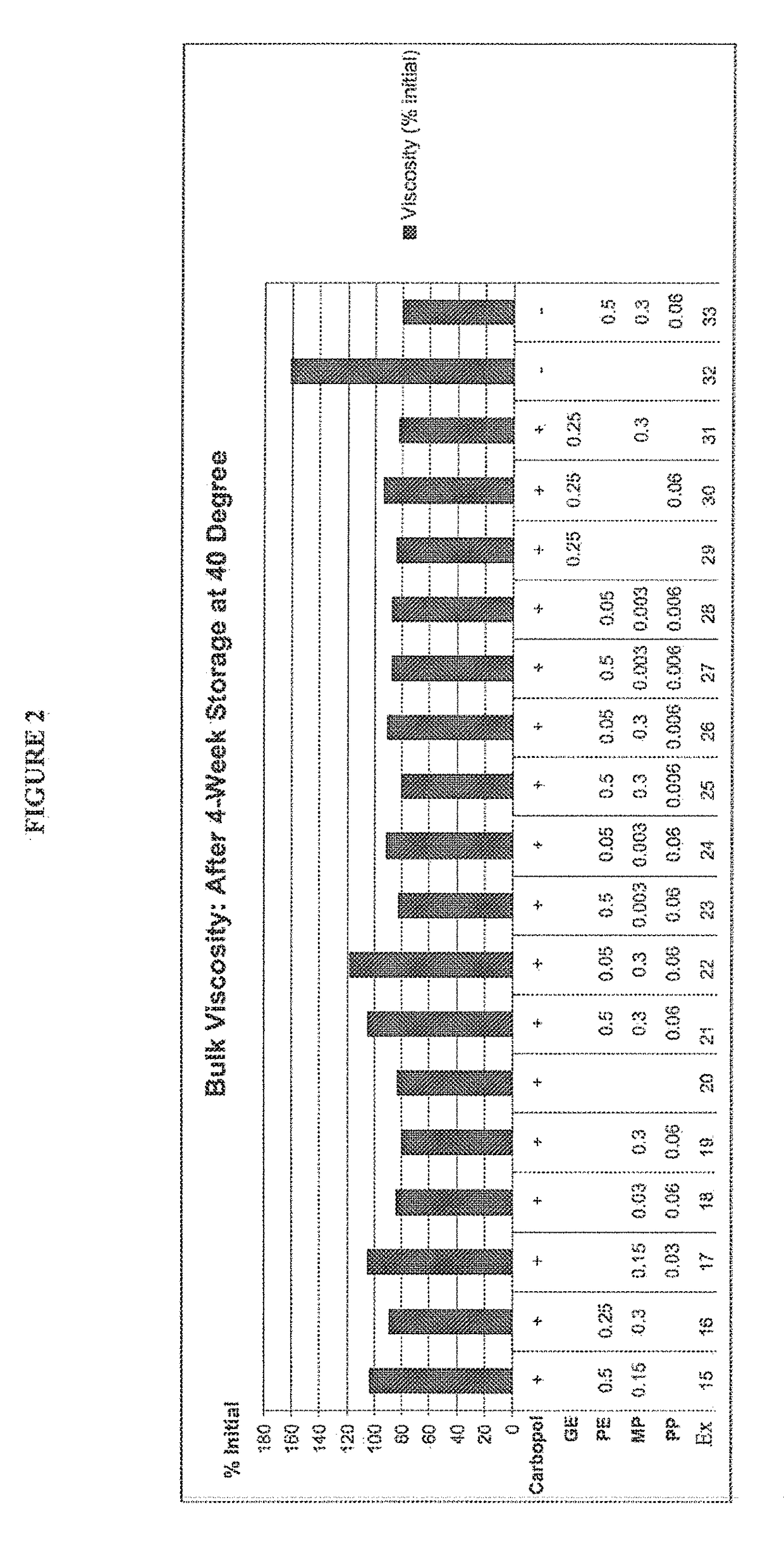
Patents
Literature
120 results about "Tretinoina" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Topical preparation for treating acne and hirsutism
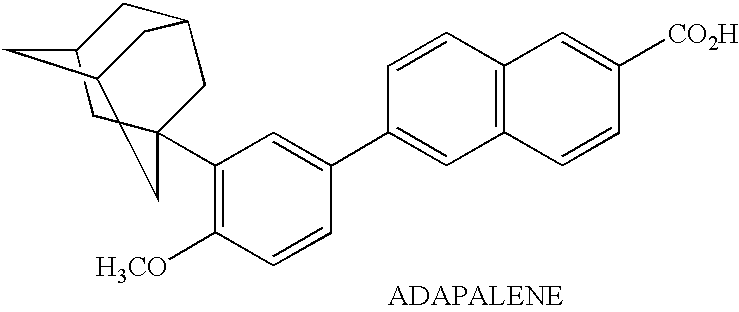
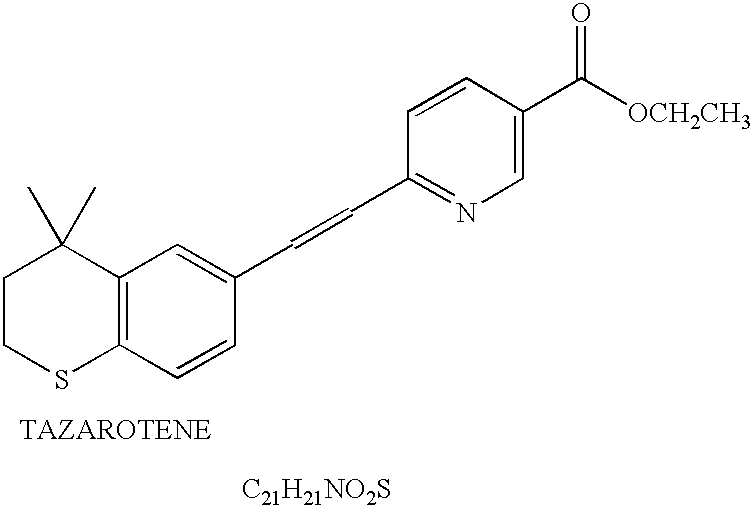
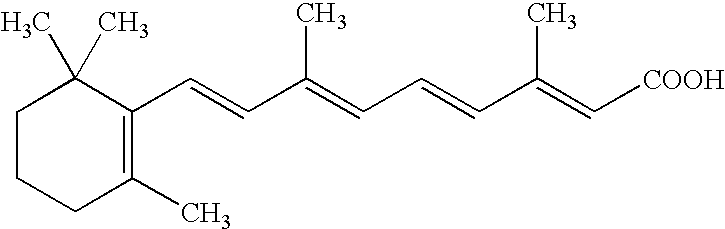
An improved method and preparation for the treatment of acne and hirsutism comprises topically applying an effective amount of a saw palmetto berry extract, in combination with two classes of low irritability penetrating agents that enhance penetration of the extract into hair follicles and sebaceous glands. The low irritability penetration agents are selected from the group (A) comedolytics consisting of adapalene, tretinoin, tretinoin gel microsponges, retinaldehyde, retinol, and tazarotene, and (B) keratinolytics consisting of the beta hydroxy acid, salicylic acid, and the alpha hydroxy acid, glycolic acid. Polyolprepolymer-2 may be incorporated into the preparation as well.
Owner:GOODMAN DAVID S
Topical aqueous composition comprising tretinoin
InactiveUS20100029765A1Increase stiffnessIncrease concentrationBiocideHydroxy compound active ingredientsTretinoinViscosity
Topical aqueous compositions for the treatment of a skin disorder particularly acne. Topical aqueous composition comprising tretinoin and a hydrophilic cellulose derivative as a gelling agent, wherein the composition has a pH of about 4 to about 6.5 and viscosity of less than about 20,000 cP or provided. The composition also relates to the topical administration of tretinoin in combination with an antibiotic.
Owner:RANBAXY LAB LTD
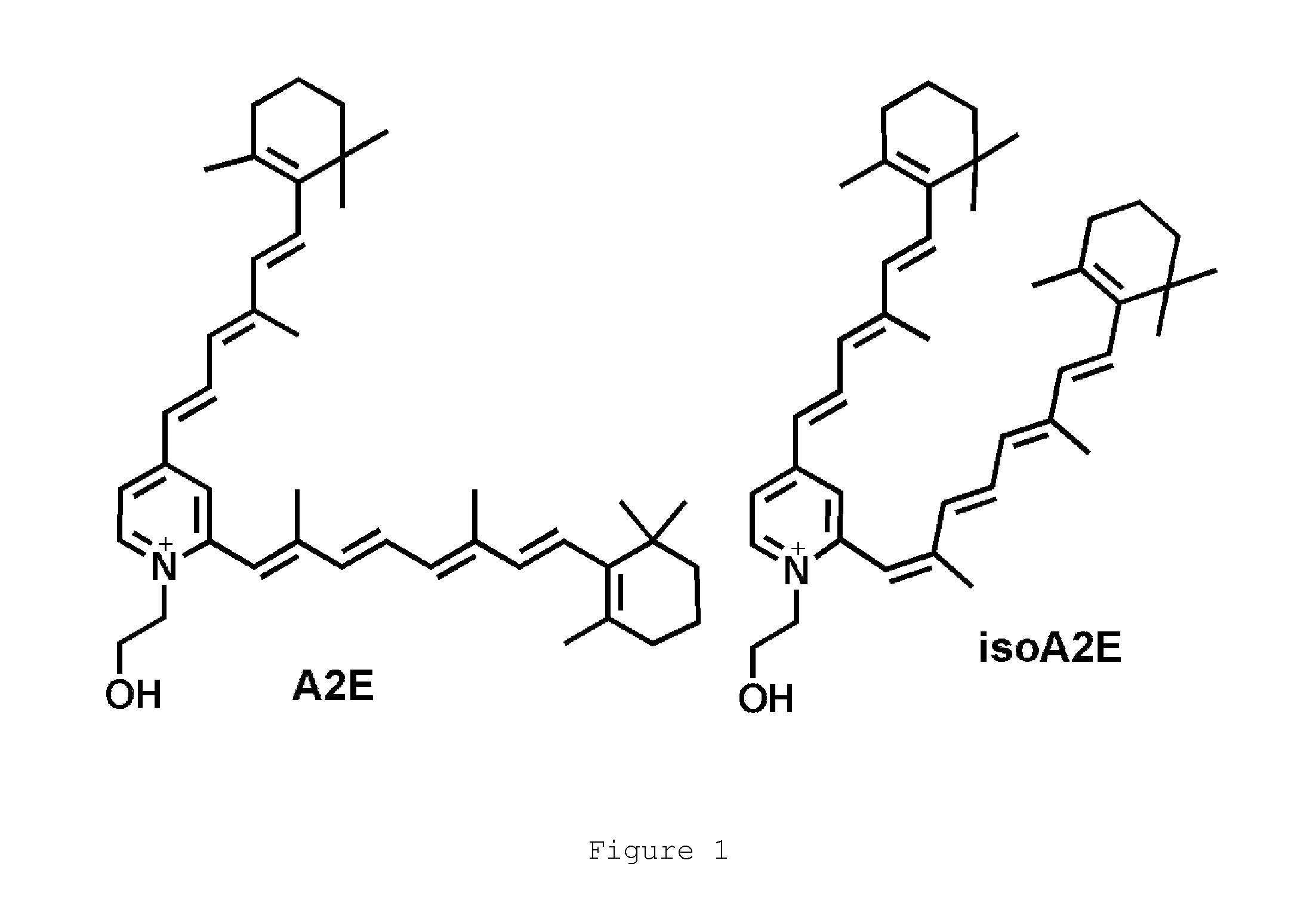
A non-retinoid rbp4 antagonist for treatment of age-related macular degeneration and stargardt disease
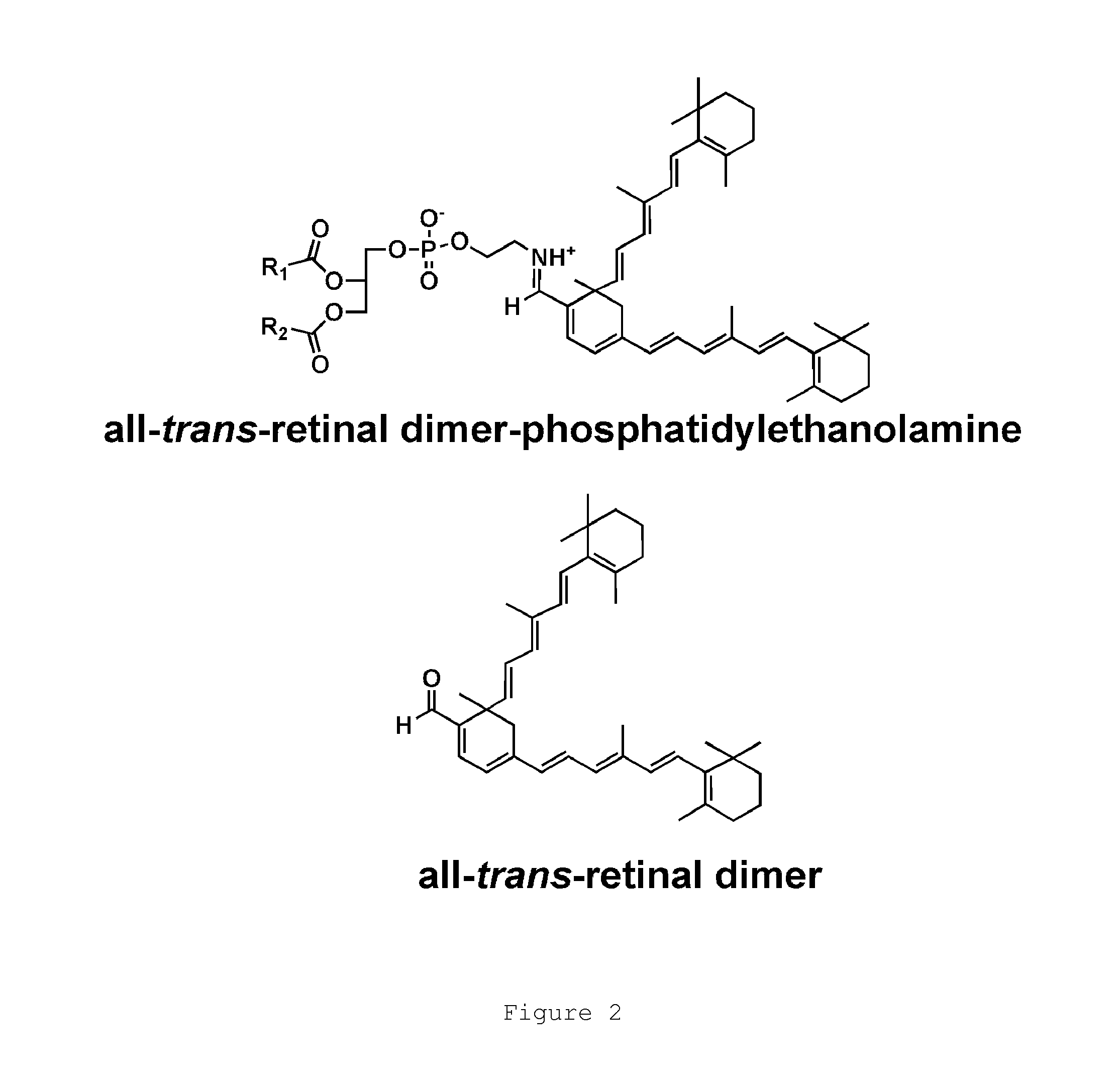
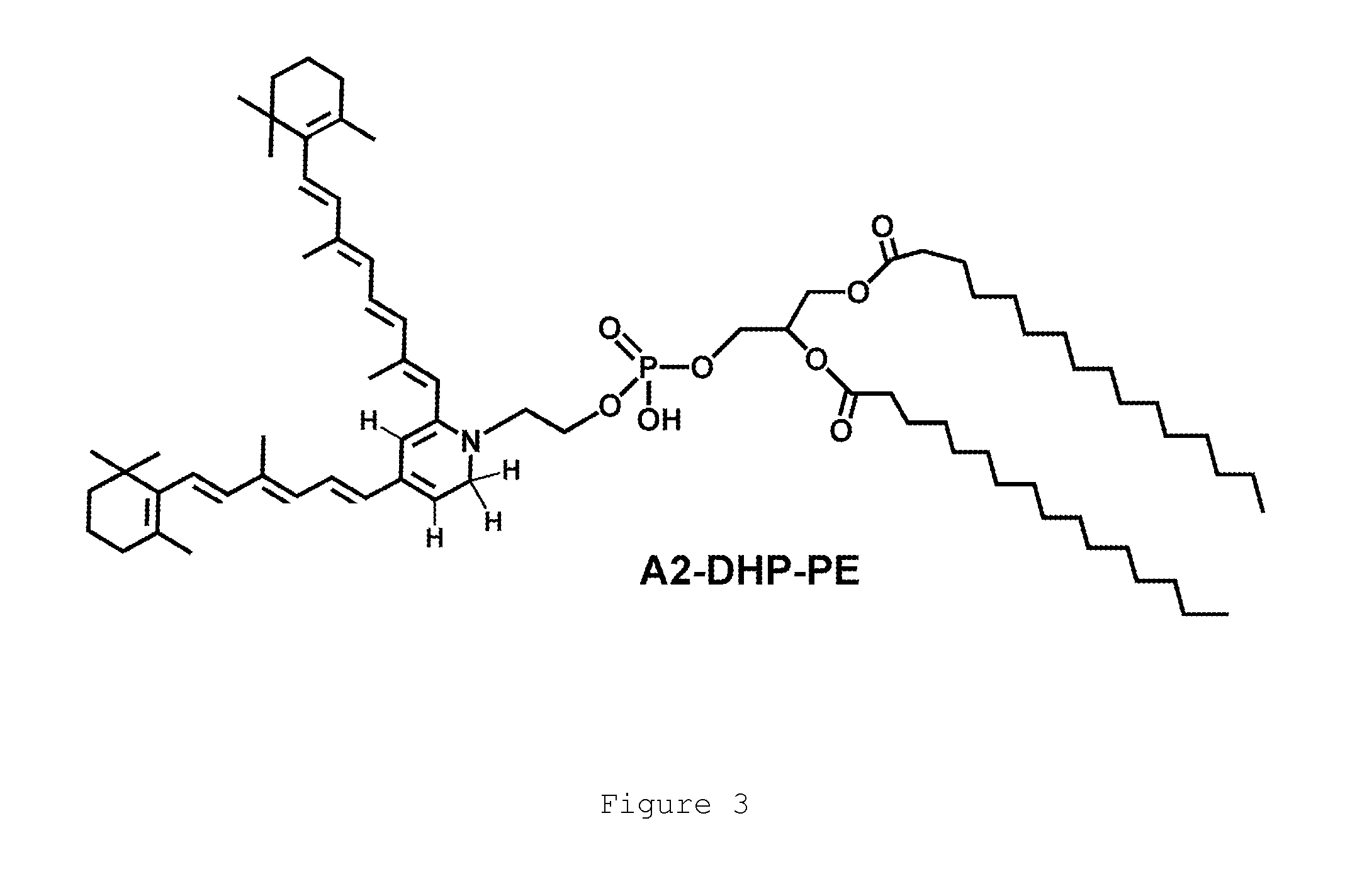
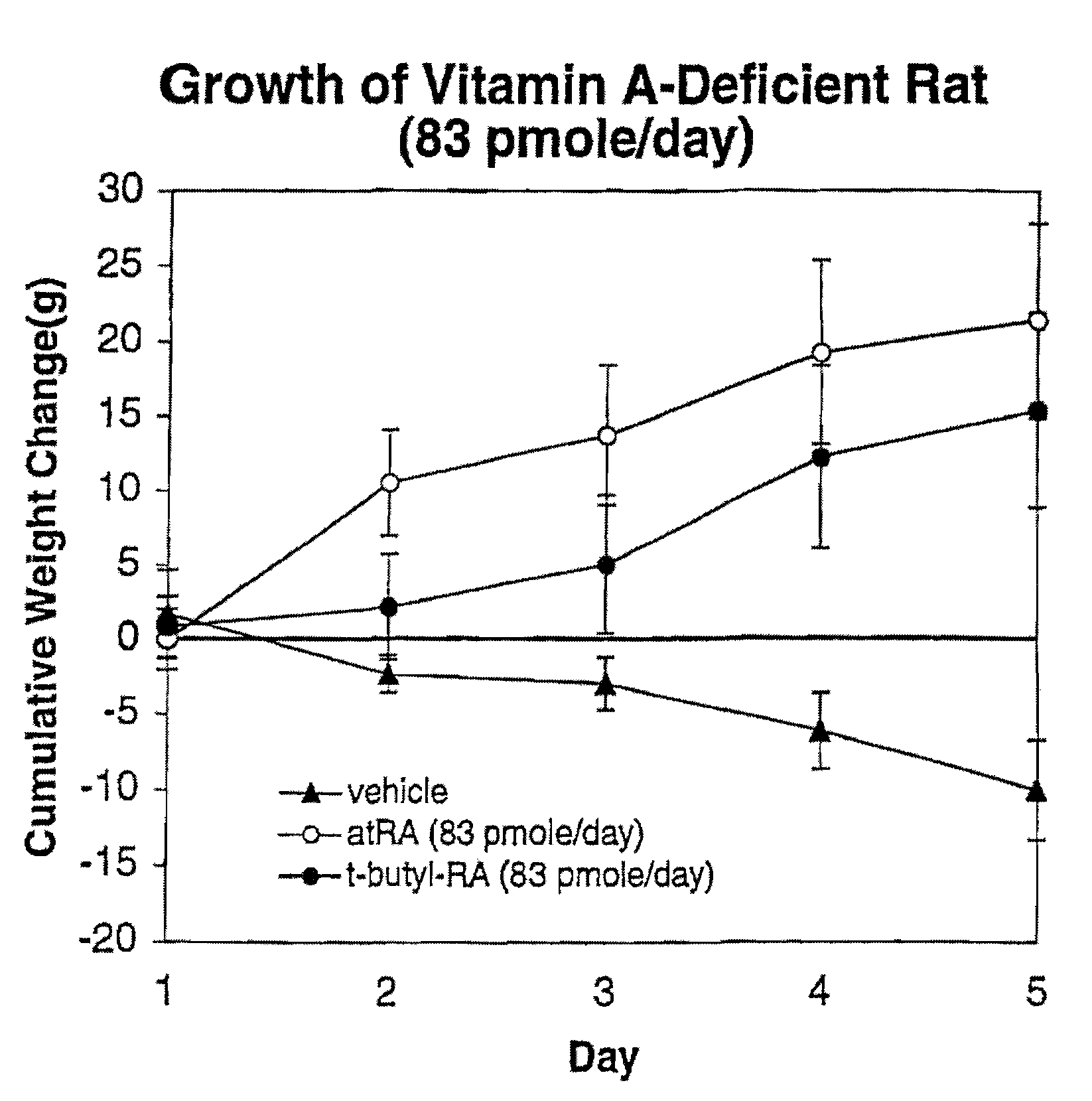
A method for treating bisretinoid-mediated macular degeneration in a mammal afflicted therewith comprising administering to the mammal an effective amount of a compound having the structure:or an ester or a pharmaceutically acceptable salt thereof.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Modified Retinoid Compounds and Their Uses
InactiveUS20080194534A1Low toxicityGreat therapeutic windowBiocideCosmetic preparationsDiseaseRetinoid
A method of minimizing or reducing the toxicity of a retinoid having a free carboxyl group and the resulting modified retinoids are described. The method comprises the step of esterifying the carboxyl group of the retinoid with a highly sterically hindered compound, which is preferably a secondary or tertiary alcohol. The resulting retinoid esters are rendered much less toxic than the starting or parent retinoid. This process provides a retinoid ester analog of reduced toxicity so that it may be administered orally with minimal side effects and with a much greater therapeutic window. The modified retinoid compounds are useful in the treatment and prophylaxis of all diseases and disorders where retinoid compounds have been shown effective.
Owner:WISCONSIN ALUMNI RES FOUND
Non-spherical drug-loaded particles and controlled release preparation of lactyl polymer and preparation methods thereof
InactiveCN101953776AStable structureNo hemolyticPowder deliveryHydroxy compound active ingredientsSolventSustained-Release Preparations
The invention relates to non-spherical drug-loaded particles and a controlled release preparation of a lactyl polymer and preparation methods thereof. The non-spherical particles of polylactic-co-glycolic acid (PLGA) are prepared by using an emulsion-solvent volatilization method assisted by small molecule materials. The PLGA is used as raw material coated with at least one of the following hydrophobic drugs: all-trans retinoic acid, paclitaxel, epirubicin, camptothecin or roxithromycin, wherein the mass ratio of the hydrophobic drug to a lactyl polymer high polymer material is 1:4-40. The drug-loaded particles of the all-trans retinoic acid are prepared and subjected to in vitro drug release evaluation. The results show that the preparation method has the advantages of simple preparation process, good reproducibility, significantly increased drug loading amount and encapsulation efficiency relative to spherical particles, very good controlled-release effect, no hemolysis initiation and safety use. The novel carrier and preparation have a potential industrial production value in the field of long-circulating controlled release of the hydrophobic drugs.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
All-trans retinoic acid-camptothecin anticancer drug conjugate as well as preparation method and application thereof
ActiveCN104478890AImprove solubilityExpand the scope of clinical applicationOrganic active ingredientsOrganic chemistryPolyoxyethylene castor oilSolubility
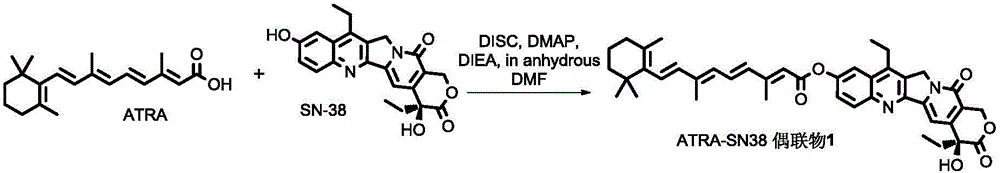
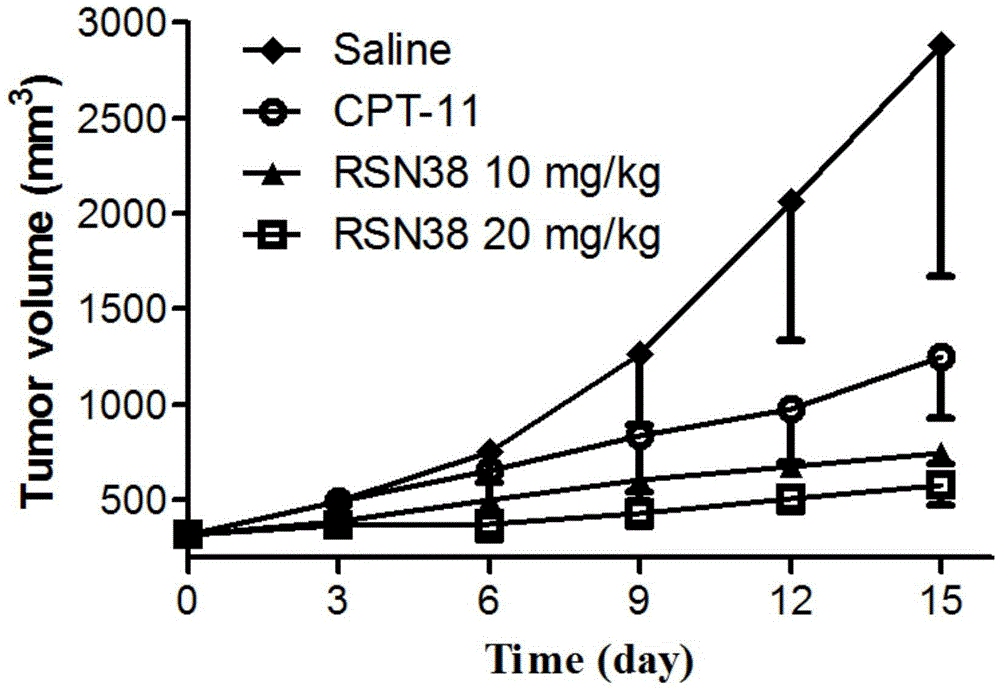
The invention discloses an all-trans retinoic acid-camptothecin anticancer drug conjugate as well as a preparation method and application thereof. The structural formula of the all-trans retinoic acid-camptothecin anticancer drug conjugate is shown in a formula (I), (II), (III), (IV), (V) or (VI). The all-trans retinoic acid-camptothecin anticancer drug conjugate has good solubility in Tween, polyoxyethylene castor oil, a Poly(ethylene adipate)-polylactic acid copolymer and a Poly(ethylene adipate)-poly (lactic acid-glycollic acid) copolymer, can be self assembled into nanometer grains in water, can be directly injected or taken orally or processed into other dosage forms. According to the all-trans retinoic acid-camptothecin anticancer drug conjugate disclosed by the invention, as all-trans retinoic acid and SN-38 or camptothecin take synergistic effect, compared with a conjugate only containing one of irinotecan, SN-38 and all-trans retinoic acid, the all-trans retinoic acid-camptothecin anticancer drug conjugate has good tumor suppression effect.
Owner:ZHEJIANG UNIV
Composite liposome of vitaminaacid as well as preparation method and application
InactiveCN1660059AEasy to operateSuitable for controlDermatological disorderAnhydride/acid/halide active ingredientsTretinoinPigmentations
Owner:CHONGQING HUAPONT PHARMA
Preparation process for insulin-secreting cells and special medium composition used therein
InactiveCN103184186ANo importEasy to operateArtificial cell constructsArtificially induced pluripotent cellsINSULIN USEInsulin Secreting Cell
The invention discloses a medium composition for inducing mesenchymal stem cells into insulin-secreting cells. The medium composition comprises a medium A, a medium B and a medium C. The medium A comprises the following solutes: a fetal calf serum with a concentration of 4.75 to 5.25 ml / L and activin A with a concentration of 4.75 to 5.25ng / mL. The medium B comprises the following solutes: retinoic acid with a concentration of 0.95 * 10<-5> to 1.05 * 10<-5> mol / L, EGF with a concentration of 19 to 21ng / ml, bFGF with a concentration of 19 to 21ng / ml, glutamine with a concentration of 1.9 to 2.1 mmol / L, 0.95 to 1.05% of non-essential amino acids and 1.9 to 2.1% of B27. The medium C comprises the following solutes: a fetal bovine serum with a concentration of 4.5 to 5.5 ml / L, exendin-4 with a concentration of 19 to 21 ng / ml, activin A with a concentration of 9.5 to 10.5 ng / ml, nicotinamide with a concentration of 9.5 to 10.5 mmol / L, 1.9 to 2.1% of B27 and 0.95 to 1.05% of N2. An induction culture method provided by the invention is applicable to a variety of tissue-derived mesenchymal stem cells and has the advantages of no introduction of viruses, easy operation and high efficiency.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
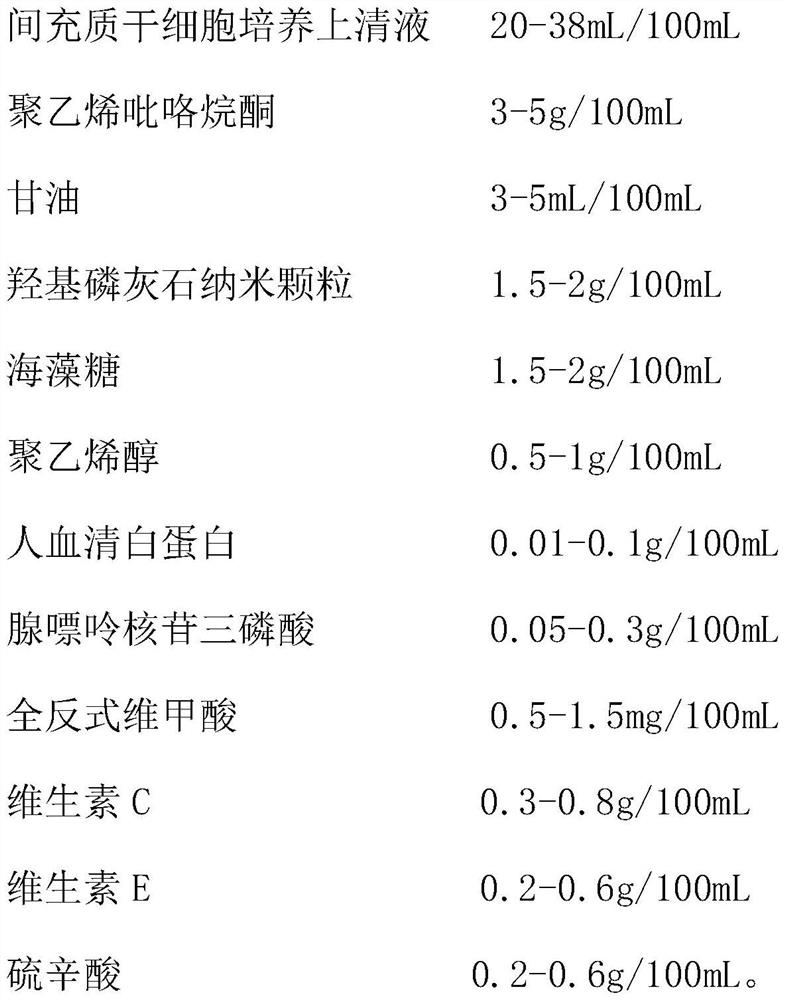
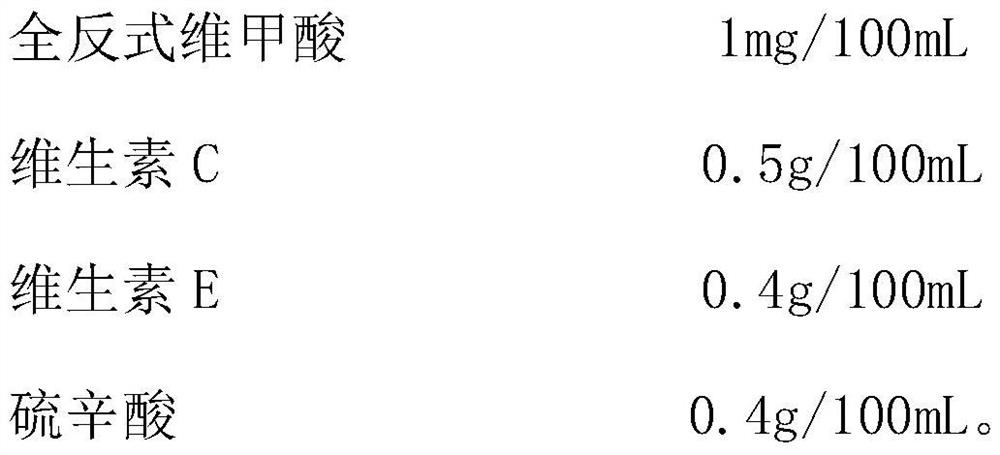
Mesenchymal stem cell cryopreservation liquid, cryopreservation method, preservation kit and thawing method
ActiveCN111011363AAvoid damageHigh activityDead animal preservationSkeletal/connective tissue cellsPolyvinyl alcoholMedicine
The invention provides a mesenchymal stem cell cryopreservation liquid, a cryopreservation method, a preservation kit and a thawing method, and relates to the technical field of cell biology. The mesenchymal stem cell cryopreservation liquid comprises the following components with the following working concentrations: 25-35 ml / 100ml of a mesenchymal stem cell culture supernatant; 4-6 ml / 100ml of glycerol, 4-6 ml / 100ml of polyvinylpyrrolidone, 0.5-1.5 g / 100ml of polyvinyl alcohol, 0.5-2.5 g / 100mL of trehalose, 1-2.5 g / 100mL of hydroxyapatite nanoparticles, 0.5-1.5 mg / 100mL of all-trans retinoicacid and 8-12 ml / 100ml of a serum substitute; the mesenchymal stem cell culture supernatant is prepared by the following method: culturing mesenchymal stem cells at the convergence degree of 78-82% for 22-26 hours, filtering, and retaining a filtrate, thereby obtaining the mesenchymal stem cell culture supernatant. The cryopreservation liquid can effectively reduce damage to cells in cryopreservation and resuscitation processes and improve the activity of the resuscitated cells.
Owner:GUANGDONG VITALIFE BIOTECHNOLOGY CO LTD
Probiotics and application thereof in secondary osteoporosis
ActiveCN111621449AStimulus formationImproved expression of osteogenic marker genesBacteriaSkeletal disorderBiotechnologyProbiotic bacterium
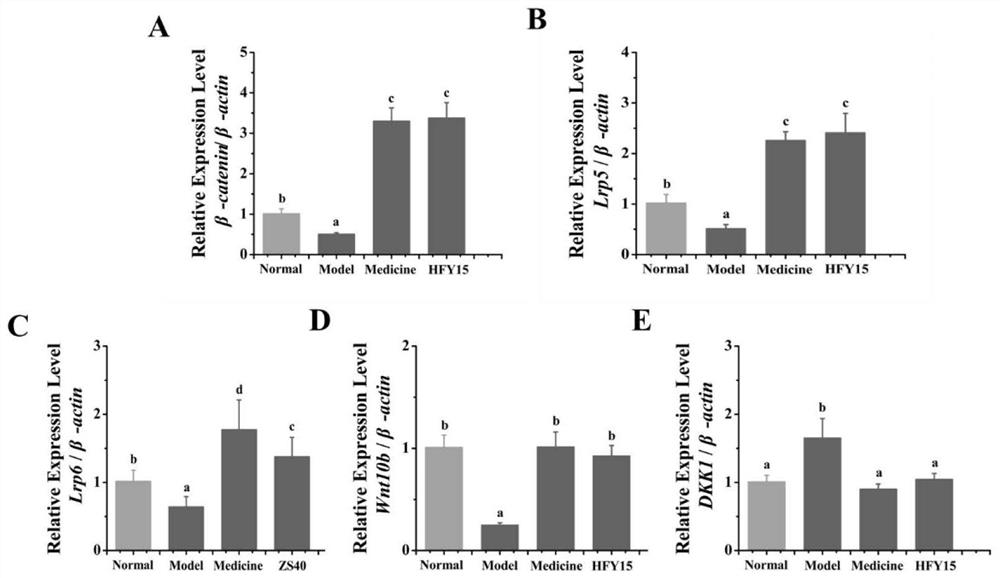
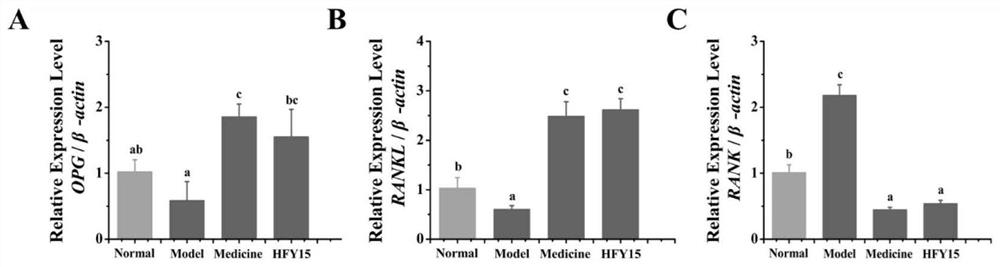
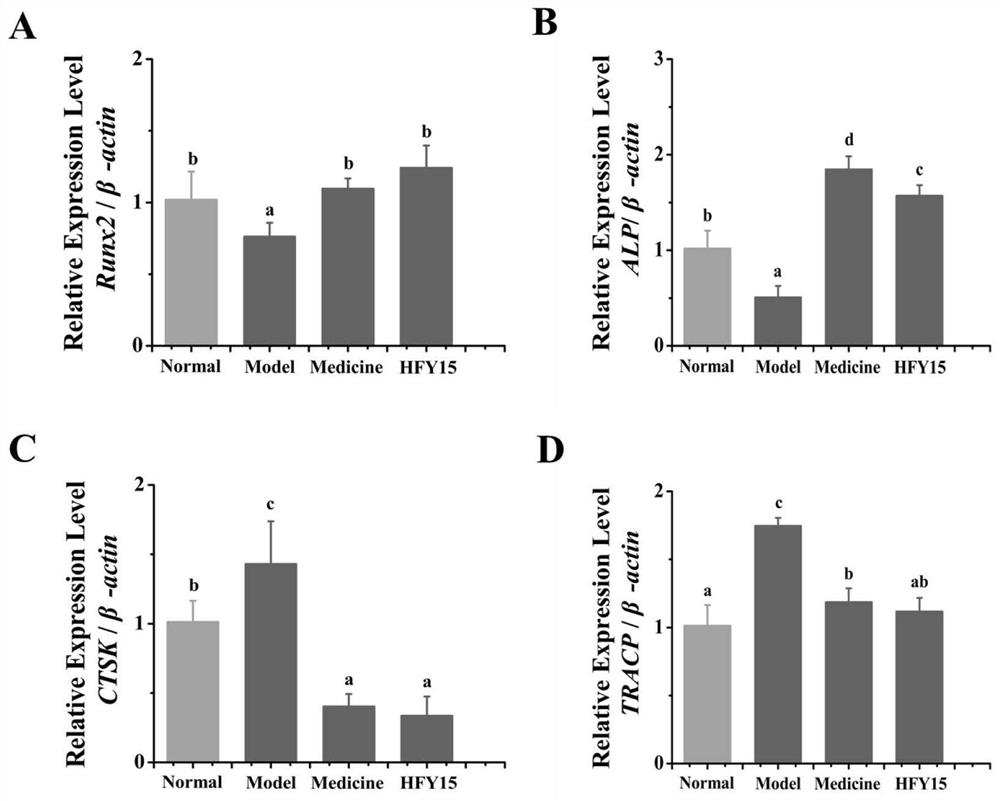
The invention discloses probiotics and application thereof in secondary osteoporosis, and belongs to the technical field of microorganisms. The lactobacillus plantarum HFY15 is lactic acid bacteria (LAB) with the preservation number of CGMCC No. 16648, and the Lactobacillus plantarum HFY15 is separated and identified from natural yak yoghourt. A rat secondary osteoporosis (OP) model is establishedby using retinoic acid, and prevention effect of the ahfy15 on the OP is researched; the results show that the supplement of the HFY15 increases the expression of osteogenic marker genes, inhibits the expression of osteoclast genes, and stimulates the formation of bones; and a research result provides a new effective strategy for prevention and treatment of OP.
Owner:安徽善和生物科技有限公司
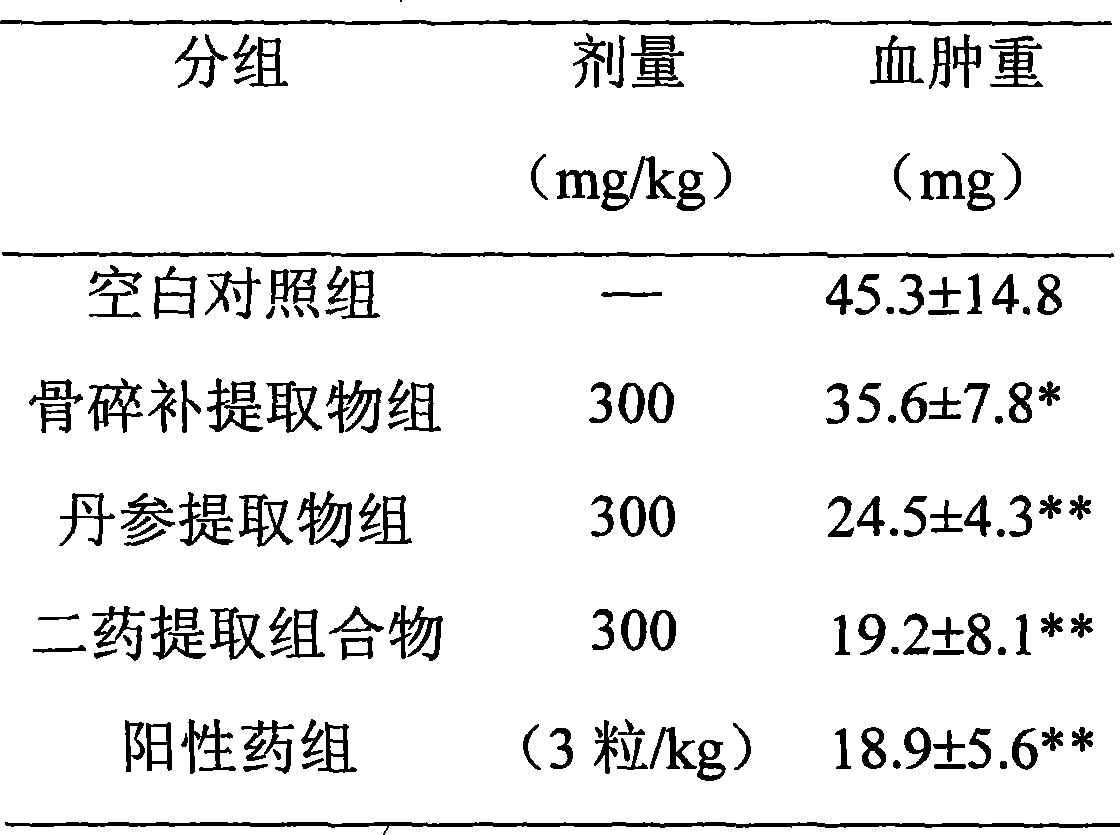
Medicament composition containing drynaria and salvia root, and use thereof
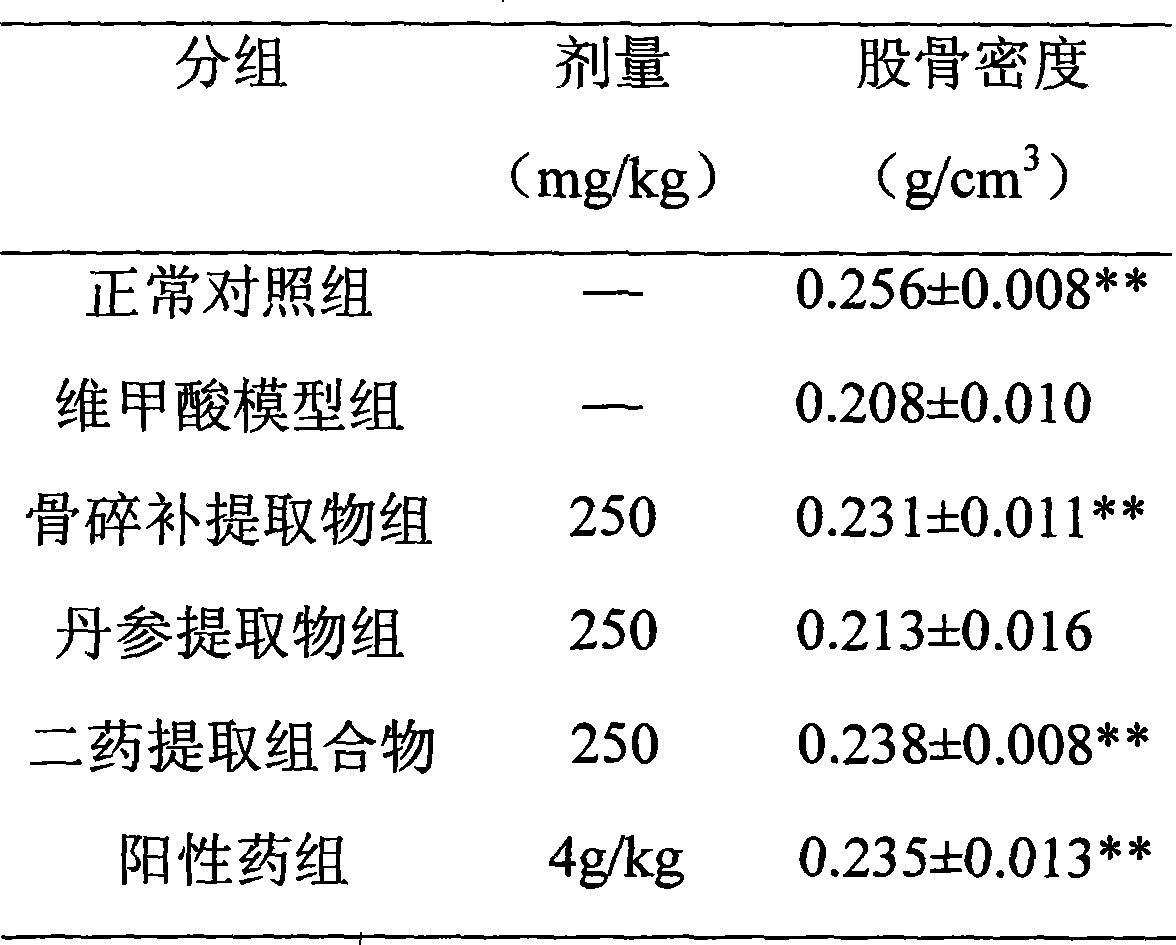
The invention relates to a medicine composition for treating osteoporosis, in particular to the medicine composition of rhizoma drynariae and radix salviae miltiorrhizae, and medicinal application thereof. The weight ratio of the rhizoma drynariae to the radix salviae miltiorrhizae is 1:0.2-10. Main active ingredients in the extract of the composition are a flavonoid compound from rhizoma drynariae, a diterpenoid tanshinone compound from radix salviae miltiorrhizae and a salvianolic acid compound, wherein contents of the three active ingredients, namely total flavonoids from the rhizoma drynariae, tanshinone and salvianolic acids, are not lower than 40 percent of total weight of the extract. The extract of the composition can effectively avoid reduction of rat bone density caused by retinoic acid, has the remarkable effect of promoting blood circulation by removing blood stasis, and can be used for preparing the medicine for preventing and treating the osteoporosis. The traditional Chinese medicine composition has the advantages of simple formulation, high content of effective ingredients, controllable quality, safety and efficiency.
Owner:NANJING NORMAL UNIVERSITY
Compound vitamin A acid gel preparation for treating acne and its preparing method
InactiveCN1850101AObvious superiorityEasy to usePharmaceutical delivery mechanismDermatological disorderGel preparationAlcohol
The present invention discloses a compound gel preparation-compound tretinoin gel preparation for curing acnes. It is made up by using carbomer, glycerine, propylene alcohol, Tween, triethanolamine, clindamycin phosphate, tretinoin, EDTA and distilled water as raw material through a certain preparation process. Said invention also provides the concrete steps of its preparation method.
Owner:李海涛
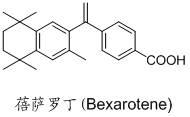
Bexarotene hydroximic acid as well as preparation method and application thereof
InactiveCN102503857AHas anti-tumor effectEasy to prepareOrganic chemistryAntineoplastic agentsHydroxizinumCancer cell
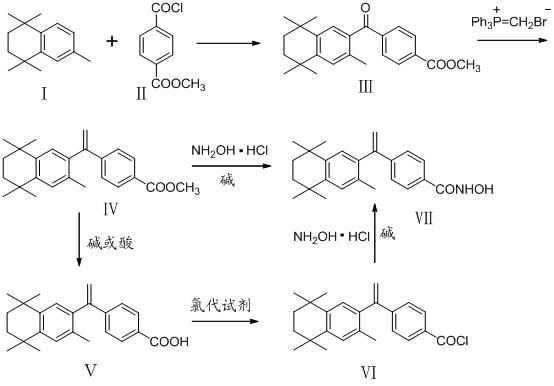
The invention belongs to the technical field of medicine and discloses bexarotene hydroximic acid as well as a preparation method and application thereof. The formation of the compound is as shown in the picture and the objective compound is prepared mainly by taking 1,1,4,4,6- pentamethyl-1,2,3,4-tetrahydronaphthalene as the initial material through the steps of Friedel-Crafts acylation, Wittig reaction and condensation reaction. The method is simple in operation, convenient in post-processing and high in yield. The objective compound has good inhibitory action on various cancer cells by the multiple action mechanisms of bonding the retinoic acid X receptors and restraining the histone deacetyltransferases, and the anti-cancer activity of the bexarotene hydroximic acid is obviously better than that of bexarotene. Therefore, the bexarotene hydroximic acid can be applied to treatment of cancers.
Owner:SHENYANG PHARMA UNIVERSITY
Compounds used for treating cancer and the use thereof
The present invention discloses compounds for the treatment of cancer and its application. These compounds comprises one of the following compound: Ammonium pyrrolidinedithiocarbamate Bay 11-7085 BIO Brefeldin A (+)-Butaclamol Calcimycin Calmidazoliur chloride Chelerythrine chloride CK2 Inhibitor 2 CGP-74514A hydrochloride CGS-12066A meleate Dequalinium dichloride Dihydroouabain Diphenyleneiodonium chloride Emetine dihydrochloride hydrate GR 127935 hydrochloride Nifedipine 6-Nitroso-1,2-benzopyrone Palmitoyl-DL-Carnitine chloride Parthenolide PD 169316 1,10-Phenanthroline monohydrate 4-Phenyl-3-furoxancarbonitrile Prazosin hydrochloride Protoporphyrin IX disodium Quinacrine dihydrochloride Quabain Retinoic acid p-hydroxyanilide Rottlerin Sanguinarine chloride Tetraethylthium disulfide and SU 9516. The invention also provides new uses of these compounds, compounds such as for the preparation of the treatment of cancer, inhibit cancer cell, cancer stem cell growth and provides a new pharmaceutical composition for treating cancers
Owner:NAT DEFENSE MEDICAL CENT
Compound used for treating acne and application thereof
The invention discloses a compound used for treating acne and an application thereof. The compound has obvious effect for treating acne, and can alleviate side effect of retinoic acid by combining with retinoic acid.
Owner:LANHE SHANGHAI BIOTECH CO LTD
Enhanced atra-related compounds for the treatment of proliferative diseases, autoimmune diseases, and addiction conditions
ActiveUS20180064666A1Improve bindingImprove curingAnhydride/acid/halide active ingredientsHeterocyclic compound active ingredientsImmunologic disordersAutoimmune condition
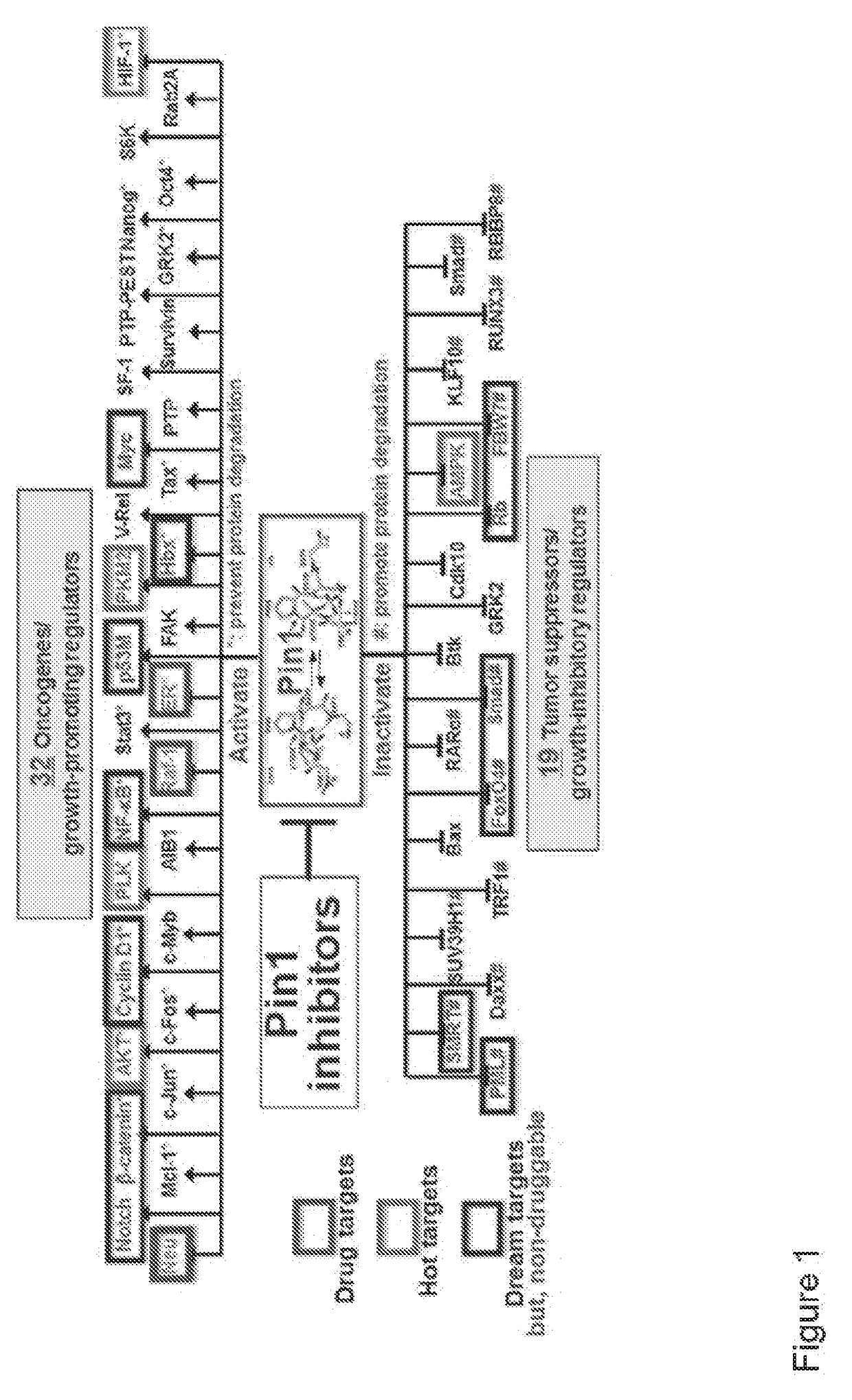
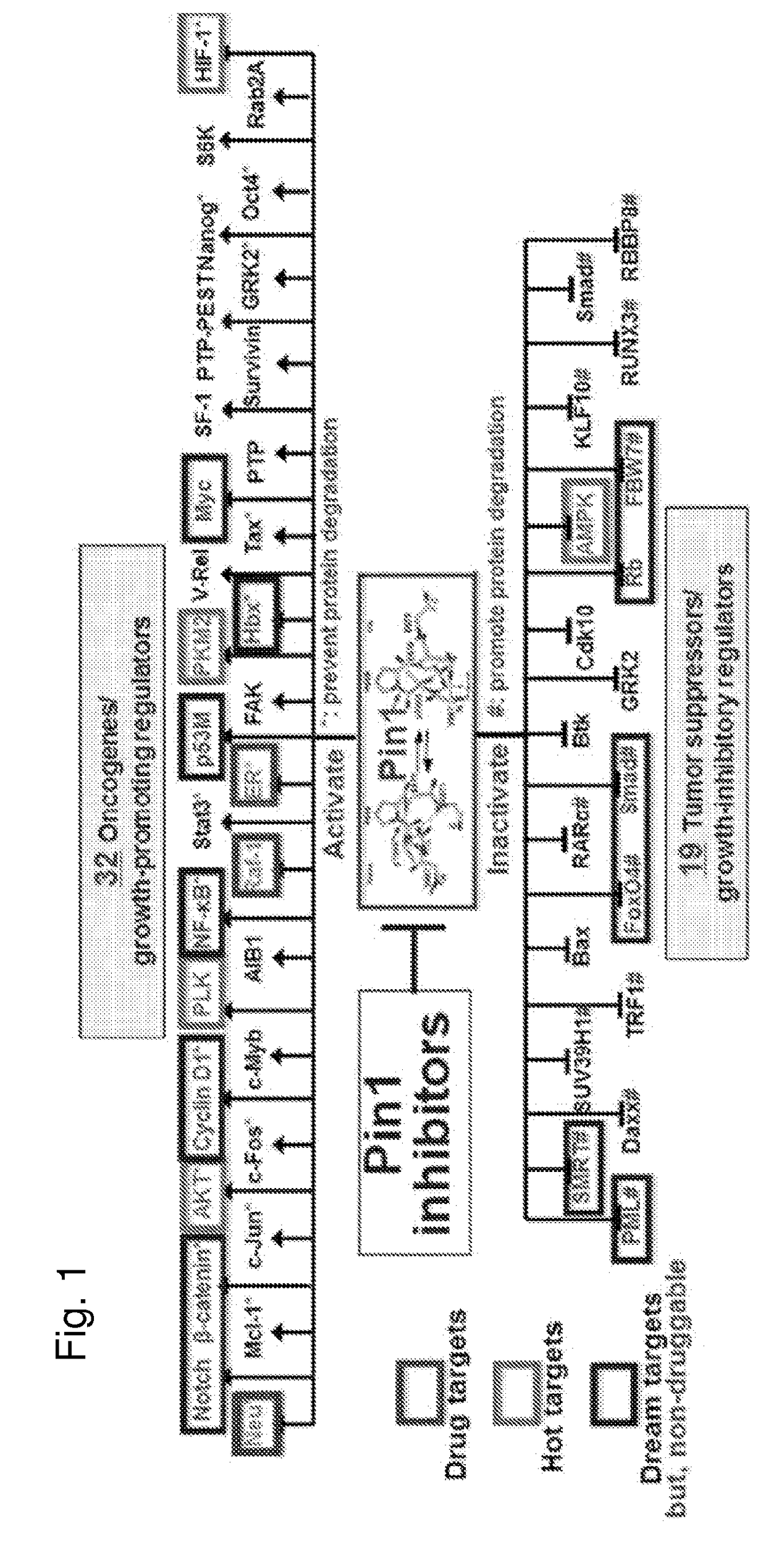
The invention features all-trans retinoic acid (ATRA)-related compounds capable of associating with Pin1 and methods of treating a proliferative disorder characterized by elevated Pin1 marker levels, Pin1 degradation, and / or reduced Pin1 Ser71 phosphorylation in a subject by administering an ATRA-related compound. The invention also features methods of treating proliferative disorders, autoimmune diseases, and addiction conditions (e.g., diseases, disorders, and conditions characterized by elevated Pin1 marker levels) by administering an ATRA-related compound in combination with another therapeutic compound.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Method for preparing retinoic hydroxyapatite bionic compound, and bionic compound
InactiveCN103948966AGreat osteogenic effectPrevention of ectopic osteogenesisPhosphorus compoundsProsthesisEctopic bone formationOrganic chemistry
The invention relates to a method for preparing a retinoic hydroxyapatite bionic compound and the bionic compound. By virtue of the method for preparing the retinoic hydroxyapatite bionic compound and the retinoic hydroxyapatite bionic compound prepared by utilizing the method, a bone is formed by utilizing the bionic compound in the clinical application, the cost is low, the effect of bone formation is good, and ectopic bone formation, postoperative swelling and other complications can be effectively avoided. The technical scheme adopted by the invention is as follows: the preparation method comprises the following steps: a, preparing a core of a bionic layer; b, coating the surface of the core of the bionic layer with a functional layer; c, coating the surface of the product obtained in the step with one more functional layer. The bionic compound is mainly applied to the technical field of medical appliances.
Owner:柳毅 +3
Enhanced atra-related compounds derived from structure-activity relationships and modeling for inhibiting pin1
InactiveUS20170112792A1Increasing and decreasing sizeIncreasing and decreasing and lengthCompound screeningOrganic chemistryDiseasePhosphorylation
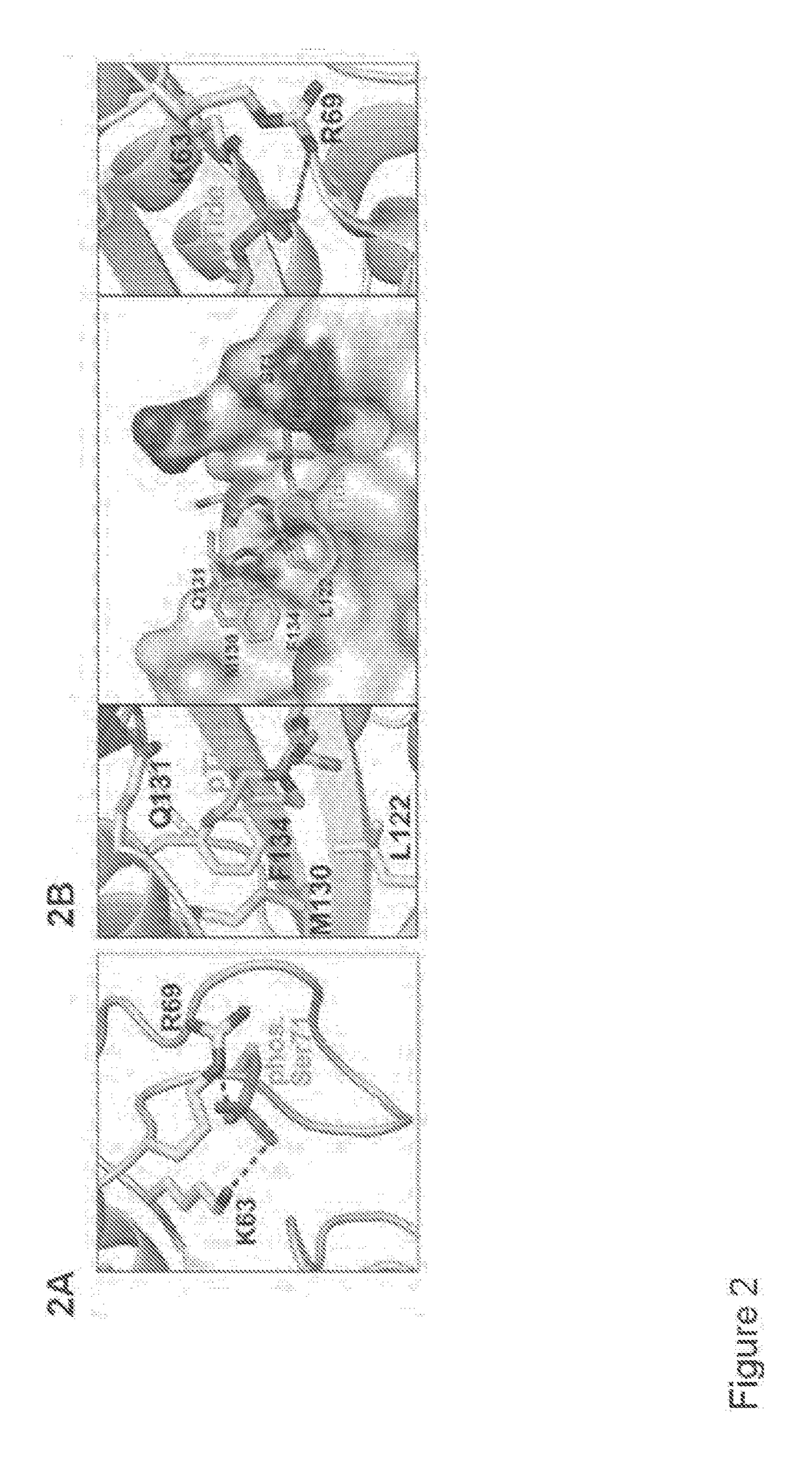
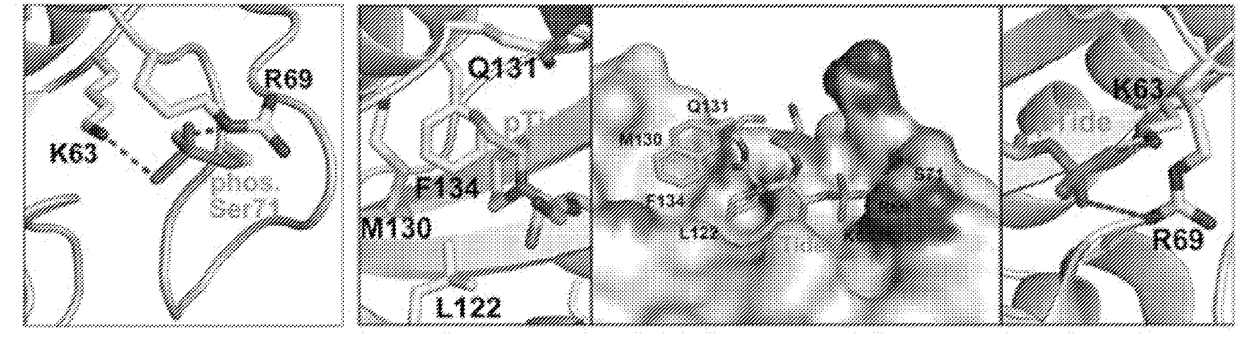
The invention features all-trans retinoic acid (ATRA)-related compounds capable of associating with Pin1 and methods of identifying the same. The invention also provides methods of treating a condition selected from the group consisting of a proliferative disorder, an autoimmune disease, and an addiction condition characterized by elevated Pin1 marker levels, Pin1 degradation, and / or reduced Pin1 Ser71 phosphorylation in a subject by administering a retinoic acid compound. Additionally, the invention features methods of treating proliferative disorders, autoimmune diseases, and addiction conditions (e.g., diseases, disorders, and conditions characterized by elevated Pin1 marker levels) by administering a retinoic acid compound in combination with another therapeutic compound. The invention also features a co-crystal including Pin1 and a retinoic acid compound. Finally, the invention also provides methods of developing and identifying enhanced Pin1-targeted ATRA-related compounds based on the newly defined unique binding pockets in the Pin1 active site revealed from the co-crystal structure, structure-activity relationship, and structural modeling.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
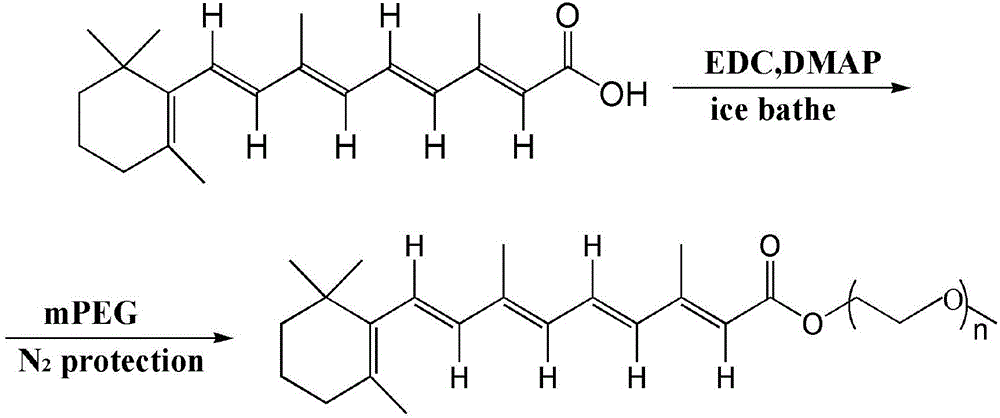
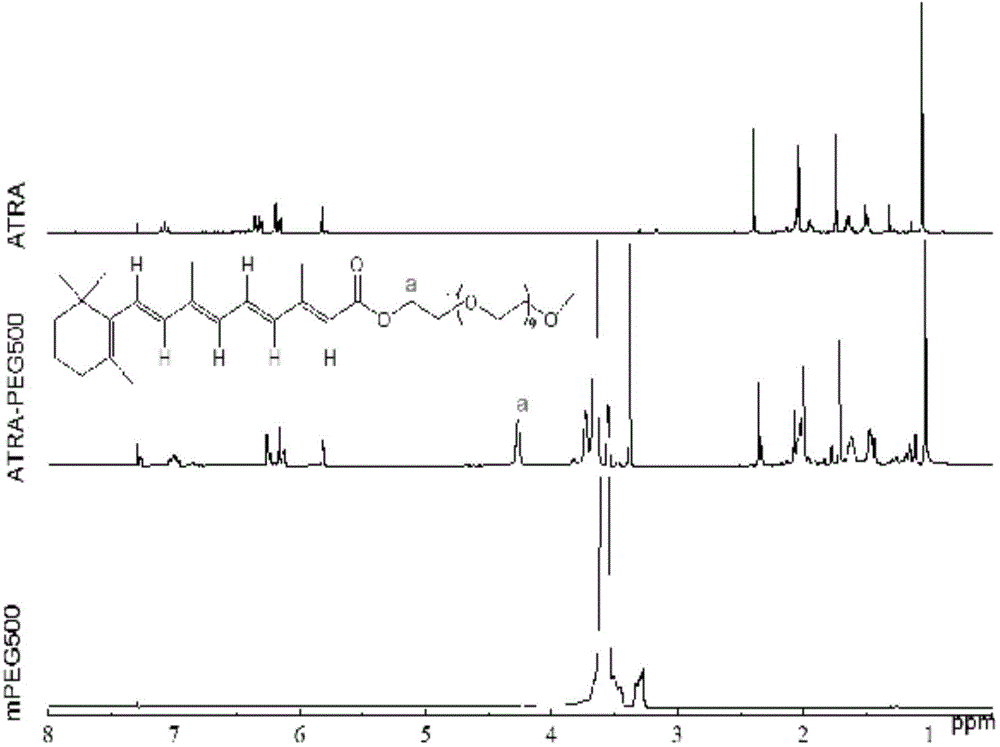
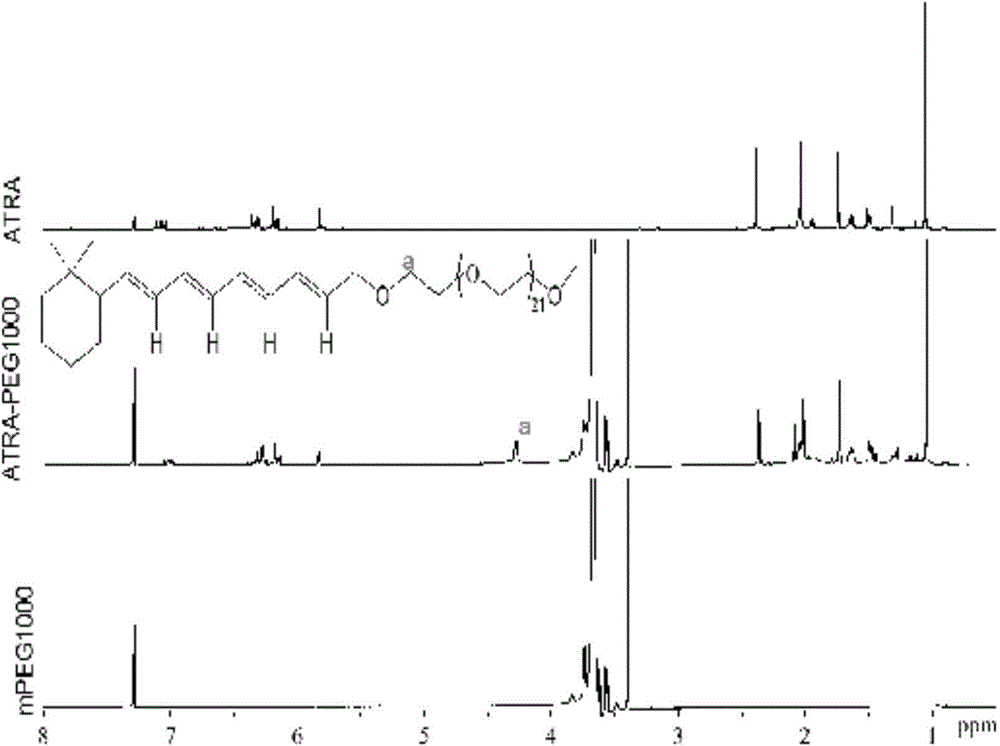
Application of pegylated retinoic acid and self-assembly micelle thereof in drug delivery
ActiveCN104399084AGentle preparationPrepared PEGylated retinoic acid prodrug block, mild carrier preparationOrganic active ingredientsPharmaceutical non-active ingredientsHalf-lifePolyethylene glycol
The invention relates to application of amphipathic pegylated retinoic acid and a self-assembly micelle thereof in drug delivery. An amphipathic prodrug block takes polyethylene glycol as a hydrophilic end and is bonded with hydrophobic retinoic acid through an ester bond, so that an AB type amphipathic prodrug block is obtained. The polymer has effects of potential treatment for leukemia and prolonging the half life of drug, has a higher drug loading rate, and effectively increases gastrointestinal mucosa permeability so as to increase the oral bioavailability. The pegylated prodrug is self-assembled to form the micelle in an aqueous medium, and the micelle can be used as a storage cavern of the indissolvable drug retinoic acid to slowly release retinoic acid. The micelle is good in safety, can be used for oral administration, and has a great market application prospect.
Owner:SHENYANG PHARMA UNIVERSITY
Emodin as inhibitor of activated molecules p-Akt and p-mTOR of PI3K/Akt/mTOR signal transduction pathway and application thereof
InactiveCN101843605ALow pricePrevent proliferationOrganic active ingredientsAntineoplastic agentsTissue proteinMTOR signaling pathway
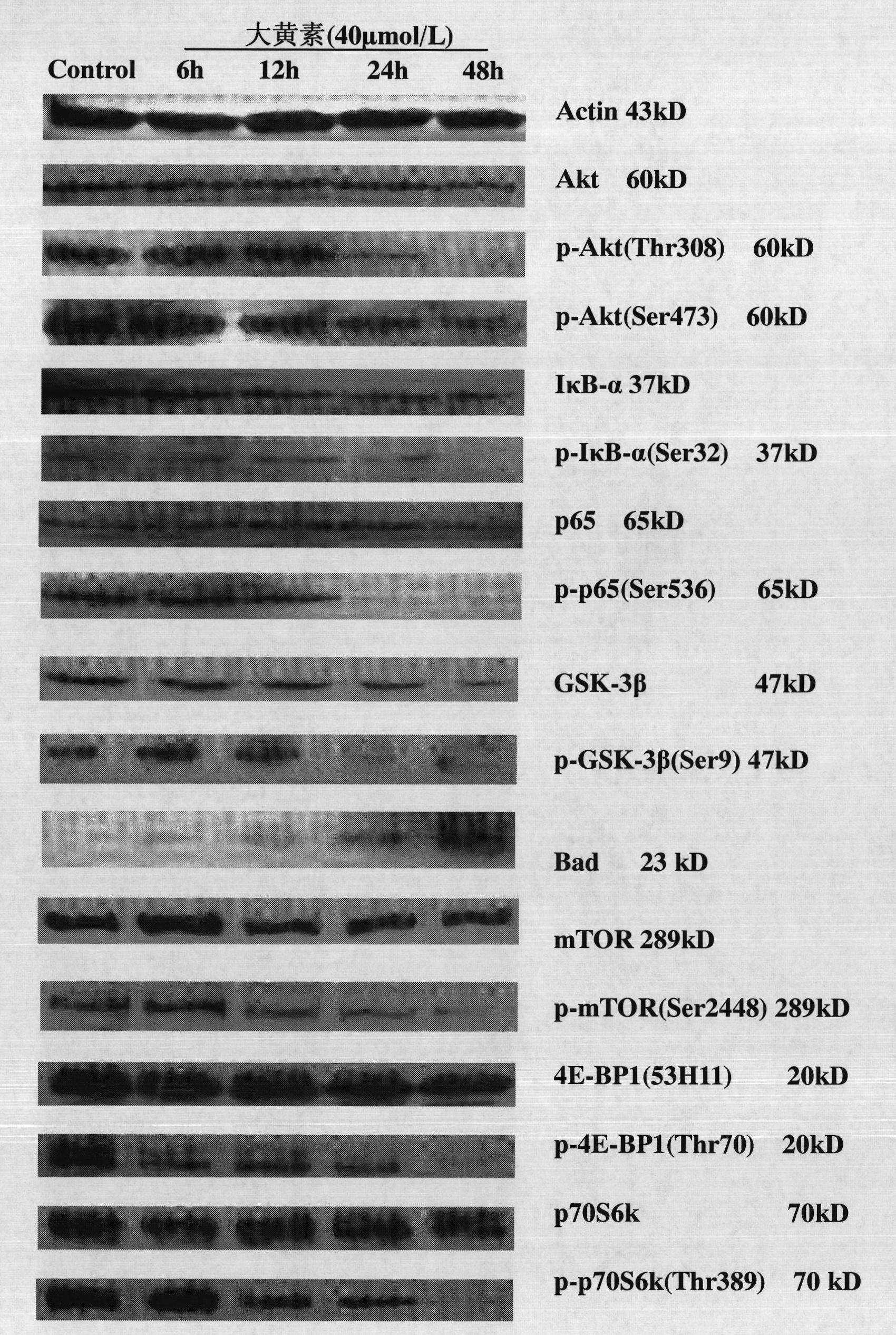
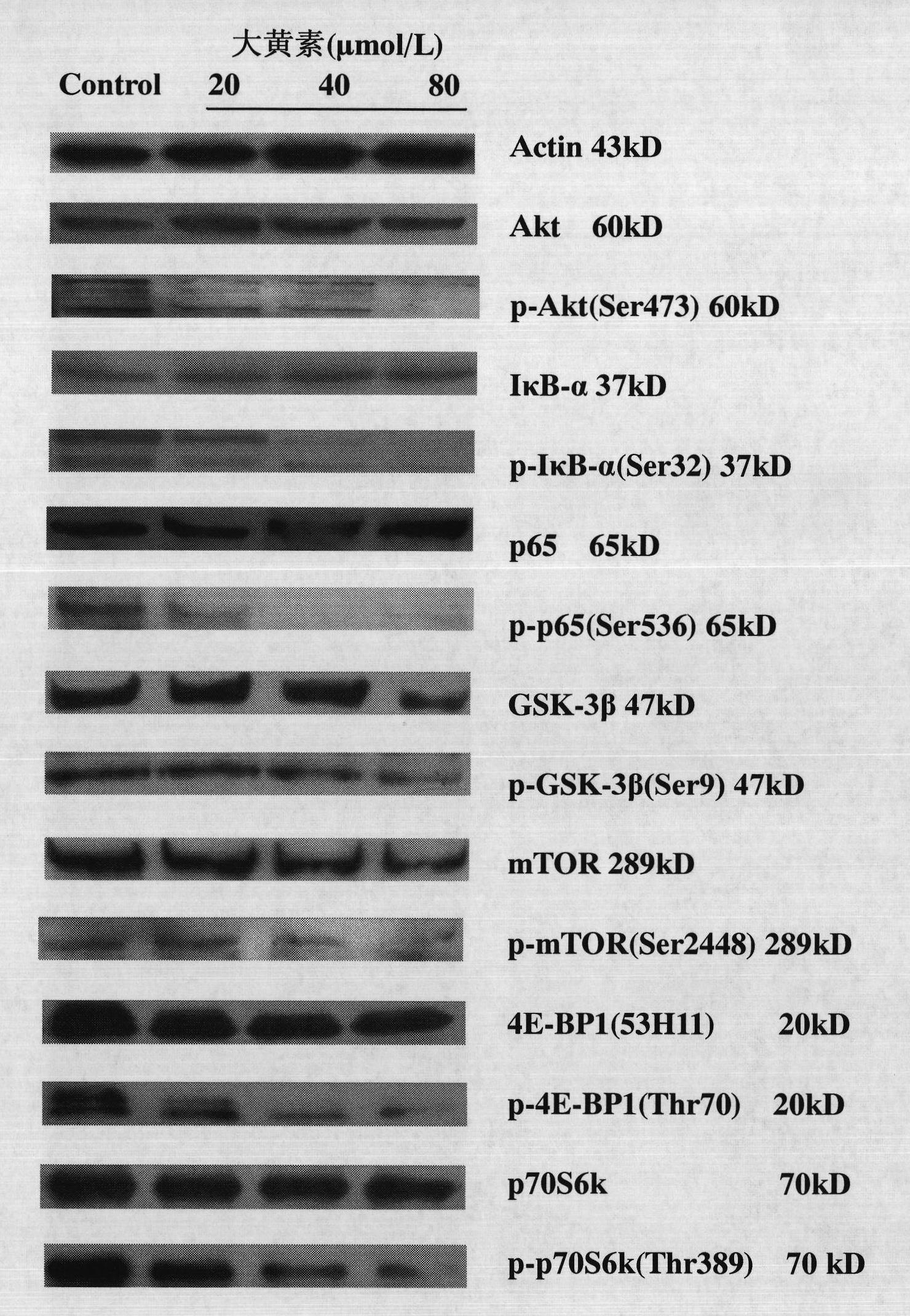
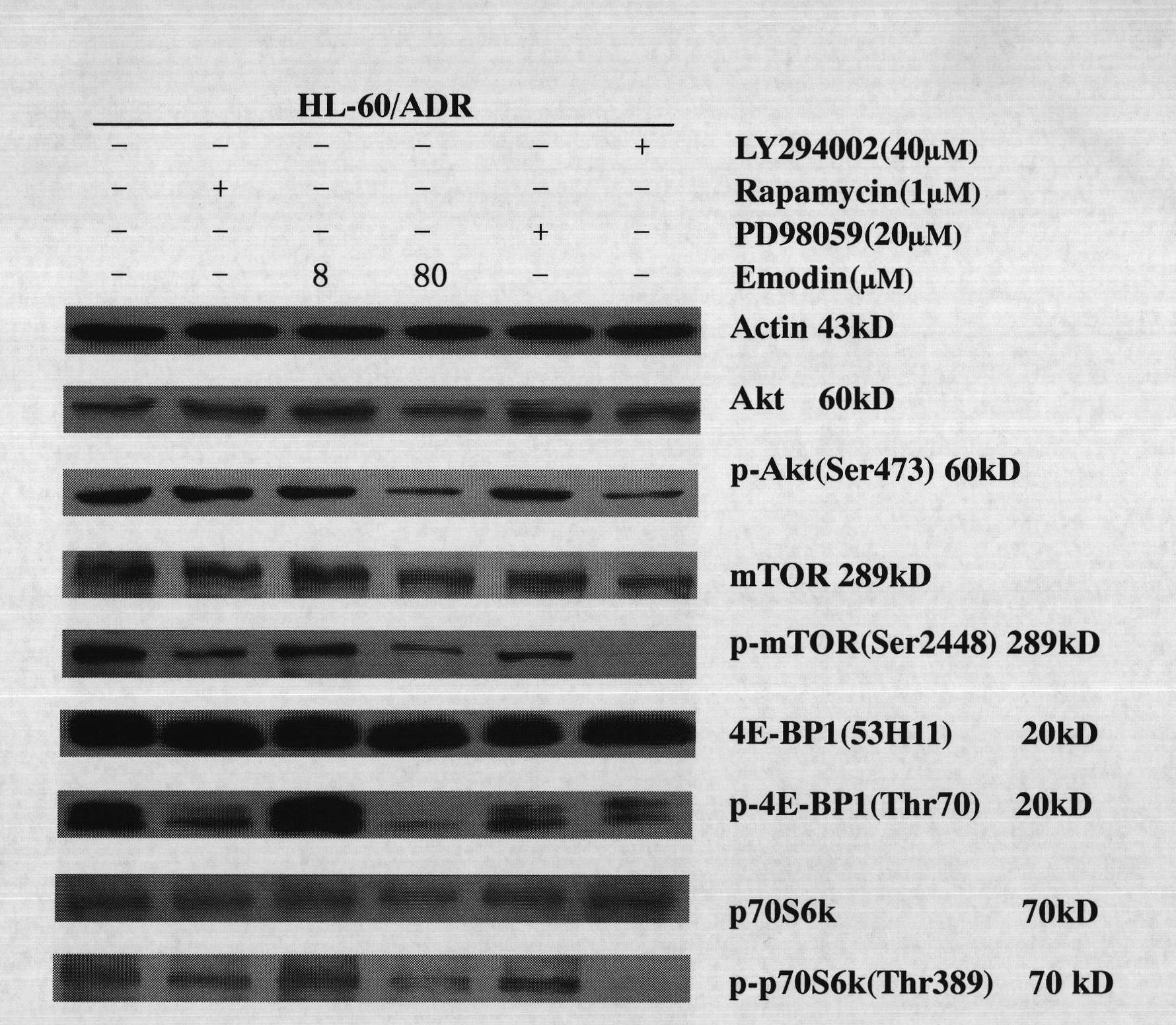
The invention discloses emodin as an inhibitor of activated molecules p-Akt and p-mTOR of a PI3K / Akt / mTOR signal transduction pathway and application thereof, wherein the emodin is a purely natural anthraquinone monomer compound extracted from rhubarb as a Chinese traditional medicine and has multiple biologic activities such as anti-microbes, anti-inflammation, antioxidation, immune regulation, liver protection and the like. The invention finds that after acute leukemia multidrug-resistance cells HL-60 / ADR, acute promyelocytic leukemia retinoic acid drug-resistant cells MR2 as well as corresponding sensitive cells NB4 and acute leukemia primary cells are acted by the rhubarb, key signal activated molecules of the PI3K / Akt / mTOR signal transduction pathway, particularly p-Akt and p-mTOR, are inhabited with specificity; in vivo researches verify that after the emodin is dosed, all the key activated molecules p-Akt, p-p65 and p-mTOR of the PI3K / Akt / mTOR signal pathway in acute leukemia nude mouse transplanted tumor tissue protein are expressed and downwards regulated, which indicates that the emodin can be used as a novel targeting inhibitor for PI3K / Akt / mTOR signal transduction activated molecules, particularly p-Akt and p-mTOR, and is applied to treating malignant tumors of a blood system.
Owner:FUJIAN MEDICAL UNIV UNION HOSPITAL
Isotretinoin amido derivative, preparation method thereof and applications thereof
ActiveCN103319365BIncrease medication optionsOvercome the technical difficulty of greatly reducing the acylation reaction activityOrganic active ingredientsOrganic compound preparationBenzoic acidDisease
The invention discloses an isotretinoin amido alkyl benzoate derivative, a preparation method thereof and applications thereof. The derivative has stronger inhibition and differentiation regulating effects than isotretinoin on psoriasis, acne and epithelial cell tumors including, but not limited to skin squamous epithelial cell carcinoma, stomach cancer, lung cancer, and cervical cancer; the derivative has less influence on normal tissue cells, has a certain targeting effect on inhibiting proliferating cells, and has very less side effects than isotretinoin; and the derivative has wide promising prophylaxis and treatment applications such as cornification abnormality diseases and cell abnormal proliferation including tumors, psoriasis, acne, and other cornification abnormality dermatopathy.
Owner:CHUGOKU IGAKU KAGAKUIN HIFUBIYOU KENKYUSHO
Method for inducing umbilical cord mesenchymal stem cells to differentiate into endothelial cells
InactiveCN102776150AArtificial cell constructsVertebrate cellsVascular tissue engineeringBiomedicine
Belonging to the field of biomedicine, the invention relates to a method for paeoniflorin to induce umbilical cord mesenchymal stem cells to differentiate into endothelial cells and application thereof. In the invention, the paeoniflorin has a structure as shown in formula (I). The induction differentiation steps of the method comprise: inducing the umbilical cord mesenchymal stem cells respectively in a beta-mercaptoethanol medium with a concentration of 1-100micromoles / L and a retinoic acid 5%DMEM medium with a concentration of 1-100micromoles / L for 24h, then shifting the cells into a paeoniflorin-containing endothelial cell conditioned medium, half of the solution is changed every two days. And after 10-14d of induction, umbilical cord mesenchymal stem cell derived endothelial cells can be obtained. In the invention, the application of the paeoniflorin and the method can promote differentiation of the umbilical cord mesenchymal stem cells, improve the differentiation efficiency, and can be used in cell transplantation treatment of ischemic vascular diseases. The obtained endothelial cells can be further prepared into cell preparations to be used for ischemic vascular disease treatment, and seed cell application of vascular tissue engineering.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Autologous adipose cell cryopreservation solution and cryopreservation method of adipose tissue-derived stromal cells and other substances
The invention relates to an autologous adipose cell cryopreservation solution and a cryopreservation method of substances such as adipose tissue-derived stromal cells. Every 100 mL of the autologous adipose cell cryopreservation solution comprises the following content components: 20 to 38 mL of mesenchymal stem cell culture supernatant, 3 to 5 g of polyvinylpyrrolidone, 3 to 5 mL of glycerinum, 1.5 to 2 g of hydroxyapatite nanoparticles, 1.5 to 2 g of trehalose, 0.5 to 1 g of polyvinyl alcohol, 0.01 to 0.1 g of human serum albumin, 0.05 to 0.3 g of adenosine triphosphate, 0.5 to 1.5 mg of all-trans retinoic acid, 0.3 to 0.8 g of vitamin C, 0.2 to 0.6 g of vitamin E and 0.2 to 0.6 g of lipoic acid. Compared with the prior art, ice crystal formation can be effectively reduced, and cell damage caused by uneven temperature conduction and sharp ice crystal formation is prevented.
Owner:乔爱军
Retinoid Derivative and Pharmaceutical Composition and Use Thereof
ActiveUS20110077298A1Limited applicationHigh recurrence rateBiocideOrganic chemistryRetinoidEsophagus Cancers
The invention relates to a retinoid derivative and pharmaceutical composition and use thereof. The compound of the invention is capable of preventing or treating hematological tumours, such as acute leukemia, chronic leukemia, multiple myeloma and lymphoma, solid tumours, such as liver cancer, rectal cancer, mammary cancer and esophagus cancer, and skin disorders, such as psoriasis and acne.
Owner:ANHUI MEDICAL UNIV +1
Application of all-trans retinoic acid to mesenchymal stem cell polarization regulation
PendingCN110894488AInhibition of differentiationCulture processSkeletal/connective tissue cellsAll-trans-TretinoinBiochemistry
The application of the invention discloses an application of all-trans retinoic acid to mesenchymal stem cell polarization regulation. The application of all-trans retinoic acid to mesenchymal stem cell polarization regulation specially comprises the application of the all-trans retinoic acid to restraining of polarization of mesenchymal stem cells to osteoblasts and adipocyte. The application research confirms that the all-trans retinoic acid can restrain the polarization of mesenchymal stem cells to the osteoblast and the polarization. The application provides a new function application of the all-trans retinoic acid based on the application, the polarization regulation of the mesenchymal stem cells is researched, and polarization potential of the mesenchymal stem cells is adjusted and controlled directionally. The invention provides a new tool and an adjusting means.
Owner:深圳市蓝思人工智能医学研究院 +1
Fluorescent Synthetic Retinoids
ActiveUS20170217893A1High yieldEfficient methodUltrasonic/sonic/infrasonic diagnosticsSenses disorderFluorescenceCombinatorial chemistry
Owner:HIGH FORCE RES
Pharmaceutical application of all-trans-retinoic acid
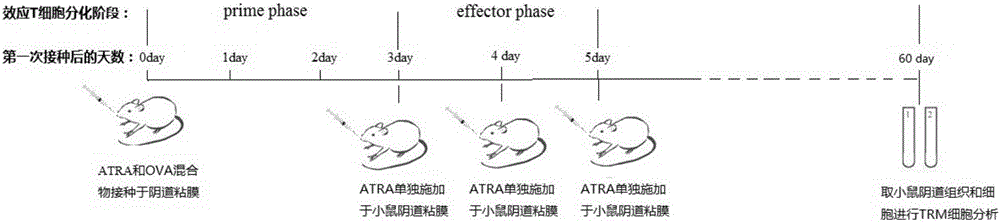
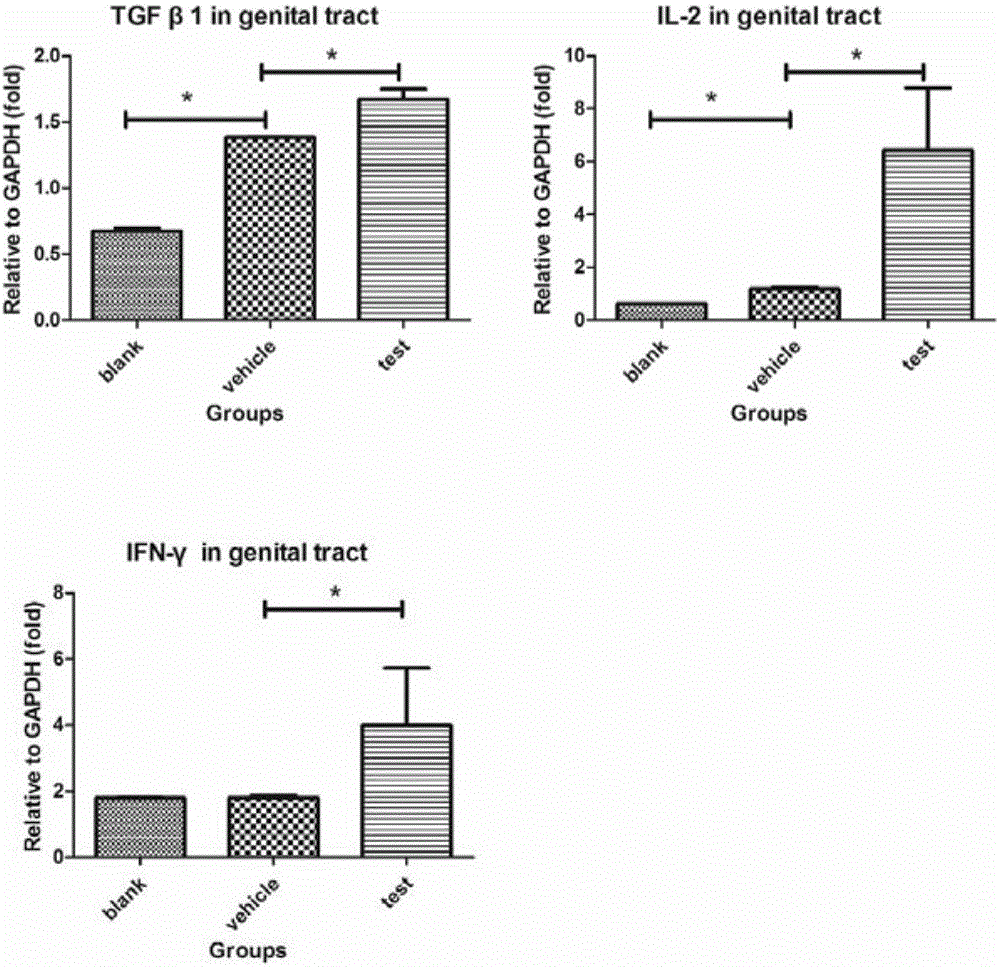
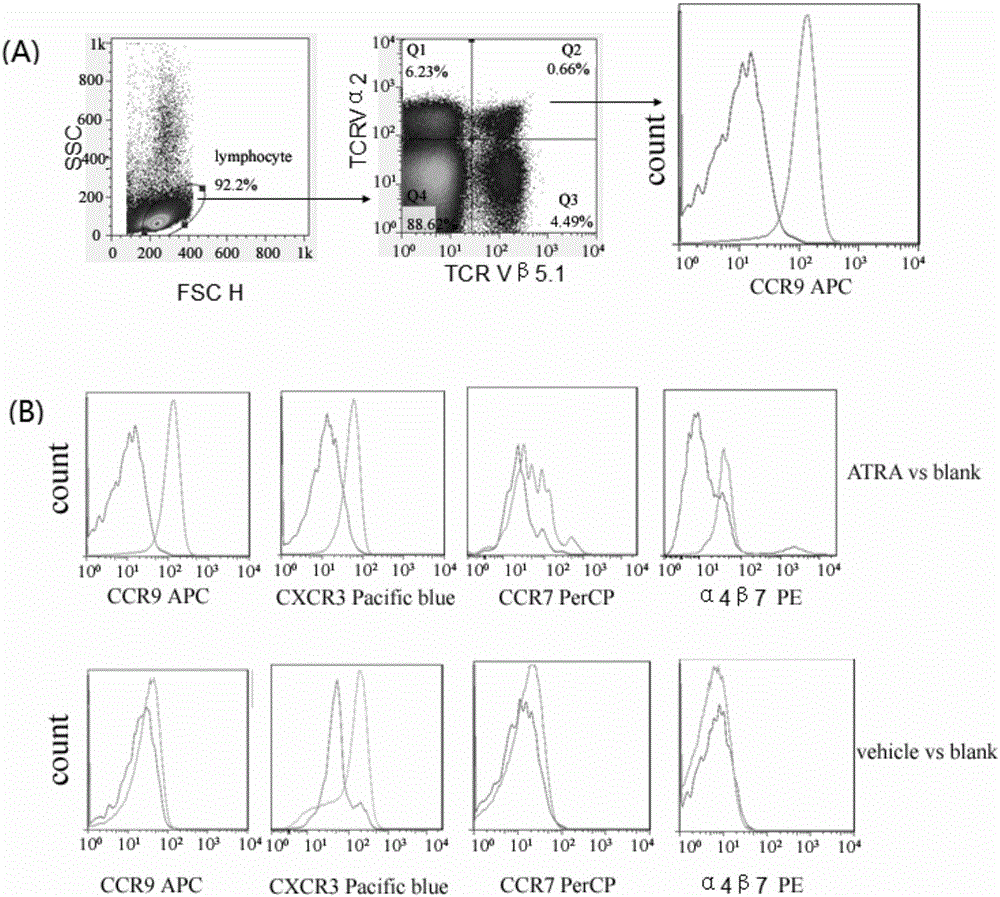
InactiveCN105748454AHydroxy compound active ingredientsImmunological disordersAdjuvantCentral Memory T-Cell
The invention belongs to the technical field of biology and particularly relates to a pharmaceutical application of all-trans-retinoic acid.By extensive and deep researches, the new application of the all-trans-retinoic acid to preparation of medicines capable of inducing formation of tissue resident memory T cells is discovered for the first time and lays a foundation for development of brand-new mucosal immunization strategies taking the ATRA (all-trans-retinoic acid) as an adjuvant.
Owner:SHANGHAI JIAO TONG UNIV
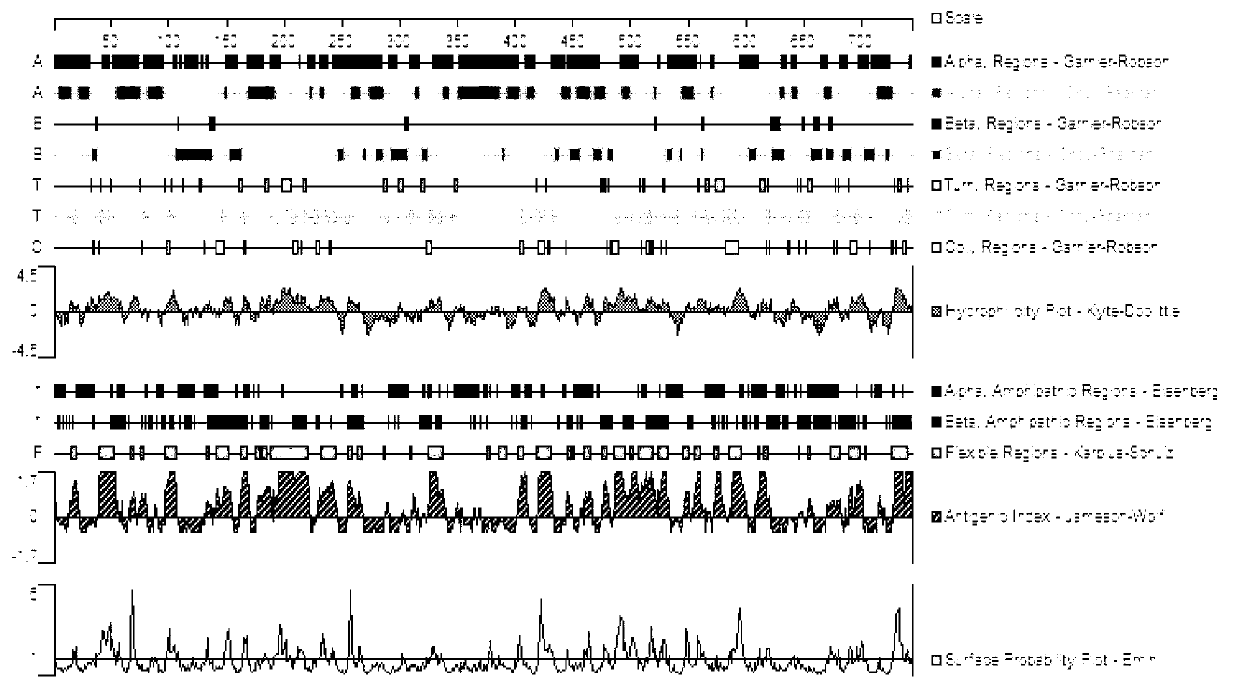
N-terminal polypeptide of retinoic acid induced protein 16 and preparation method and application of antibody thereof
InactiveCN103102392AExcellent hydrophilic structureExcellent flexibility zoneSerum immunoglobulinsImmunoglobulins against animals/humansAntigenLung cancer
The invention relates to the technical field of biomedicine and provides an N-terminal polypeptide of retinoic acid induced protein 16 (RAI16). The N-terminal polypeptide has an amino acid sequence represented by SEQ ID NO: 1 and excellent hydrophylic structures, flexible regions, antigen indexes and surface probability structures. The invention further provides an antibody of the N-terminal polypeptide of the RAI16 and a preparation method of the antibody. The antibody of N-terminal polypeptide of the RAI16, prepared by the invention, is high in valence and good in specificity and can generate a specific conjugation reaction with natural human RAI16 molecules. Furthermore, the invention provides applications of RAI16 N-terminal polypeptide and the antibody thereof in the preparation of vaccines or diagnostic kits for preventing and treating acute lymphoblastic leukemia, lung cancer, liver cancer and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method of acne treatment by concomitant topical administration of benzoyl peroxide and tretinoin
InactiveUS20190015368A1Hydroxy compound active ingredientsInorganic non-active ingredientsParticulatesSide effect
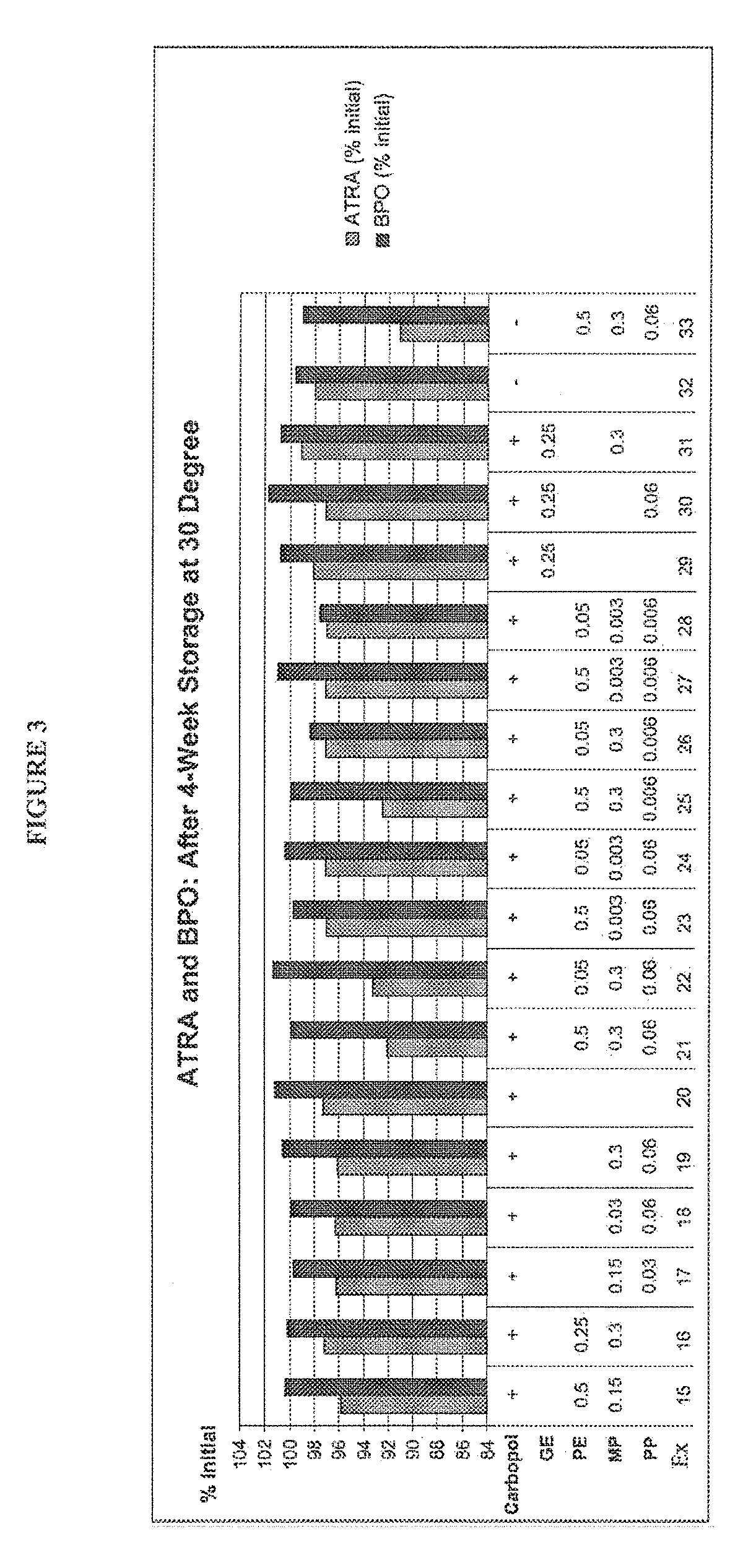
The present disclosure relates to methods of treatment of acne in a patient in need thereof, comprising the concomitant once daily topical administration of from about 2% w / w to about 10% w / w of solid particulate benzoyl peroxide (BPO) and from about 0.01% w / w to about 0.1% w / w of solid particulate all trans retinoic acid (ATRA) for a period of up to 3 months, wherein the side-effects of the two actives are medically acceptable and wherein the therapeutic effect of BPO and ATRA is superior to the effect of each active administered alone. The concomitant administration may be carried out by once daily administration to a patient in need thereof of a single composition or of a first composition comprising BPO and a second composition comprising ATRA from a dual chamber dispenser or from two separate dispensers, mixed before applying on the skin of a patient in need thereof for up to 2 weeks, up to 1 month, preferably up to 2 months and more preferably up to 3 months.
Owner:SOL GEL TECH
Administration of fluocinolone acetonide, tretinoin and hydroquinone cream in melasma maintenance therapy
InactiveUS7939514B2Reduce severityDelayed recurrenceBiocideHydroxy compound active ingredientsHypopigmentationMaintenance therapy
Topical application of a triple combination immixture of fluocinolone acetonide, tretinoin and hydroquinone is useful for the maintenance therapy of melasma to prevent hyperpigmentation recurrence or reduce the severity of the hyperpigmentation recurrence.
Owner:GALDERMA SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com