Application of pegylated retinoic acid and self-assembly micelle thereof in drug delivery
A technology of pegylated retinoic acid and retinoic acid is applied in the directions of drug combinations, medical preparations without active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of no literature report on the absorption of oral nanoparticles, etc., Achieve the effect of prolonging the half-life of the drug, simple preparation and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
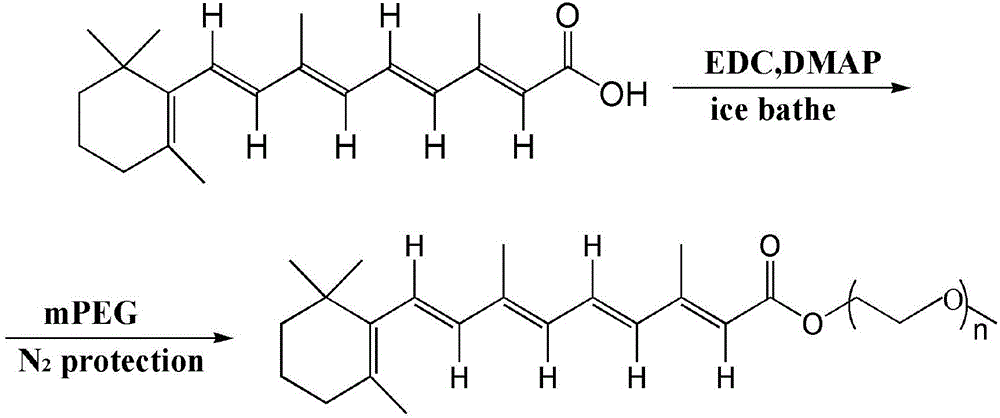
[0041] Preparation of retinoic acid prodrug blocks with different PEG chain lengths.
[0042] All-trans retinoic acid (ATRA) was dissolved in a small amount of dichloromethane in 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and 4-dimethylamino Under the catalysis of pyridine (DMAP), keep dark ice bath for 0.5-2h, then mix with polyethylene glycol (PEG) with different chain lengths at 30-40℃N 2 React for 10-12 hours under protection, and obtain a yellow oily pegylated retinoic acid amphiphilic prodrug through separation and purification. The reaction formula is as follows:
[0043]
Embodiment 2
[0045] The mPEG in the reaction formula can be mPEG 500 , mPEG 1000 , mPEG 2000 and mPEG 5000 That is, the selected polyethylene glycol has a molecular weight of 500, 1000, 2000 or 5000. The polyethylene glycol of the present invention is one end methylated modified polyethylene glycol, but it is not limited to the above four substances.
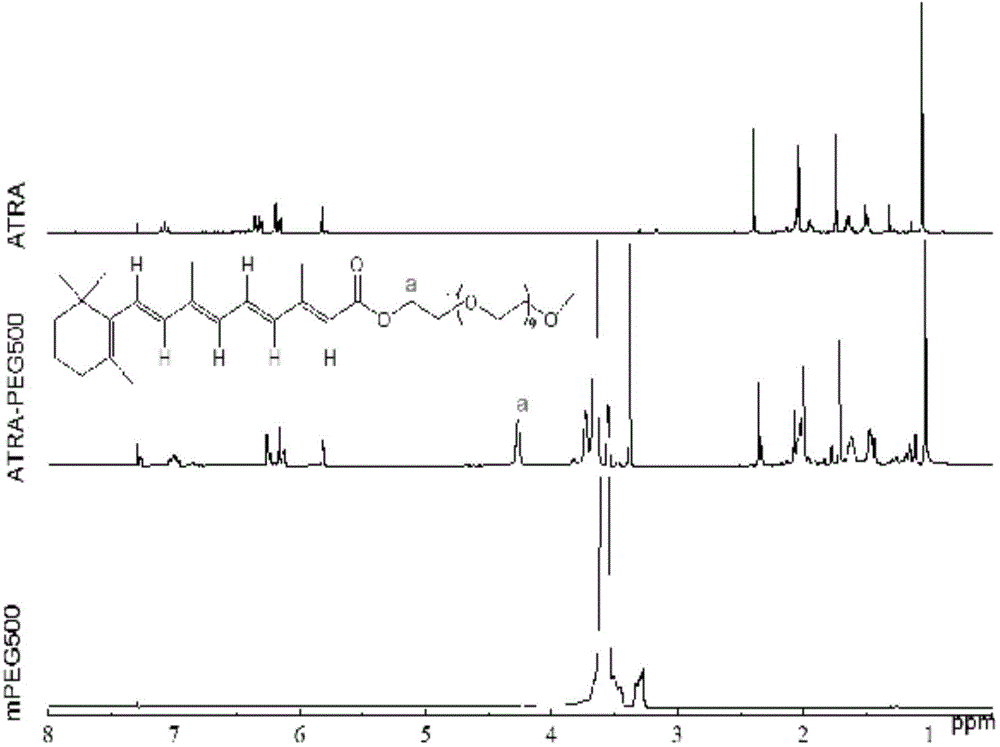
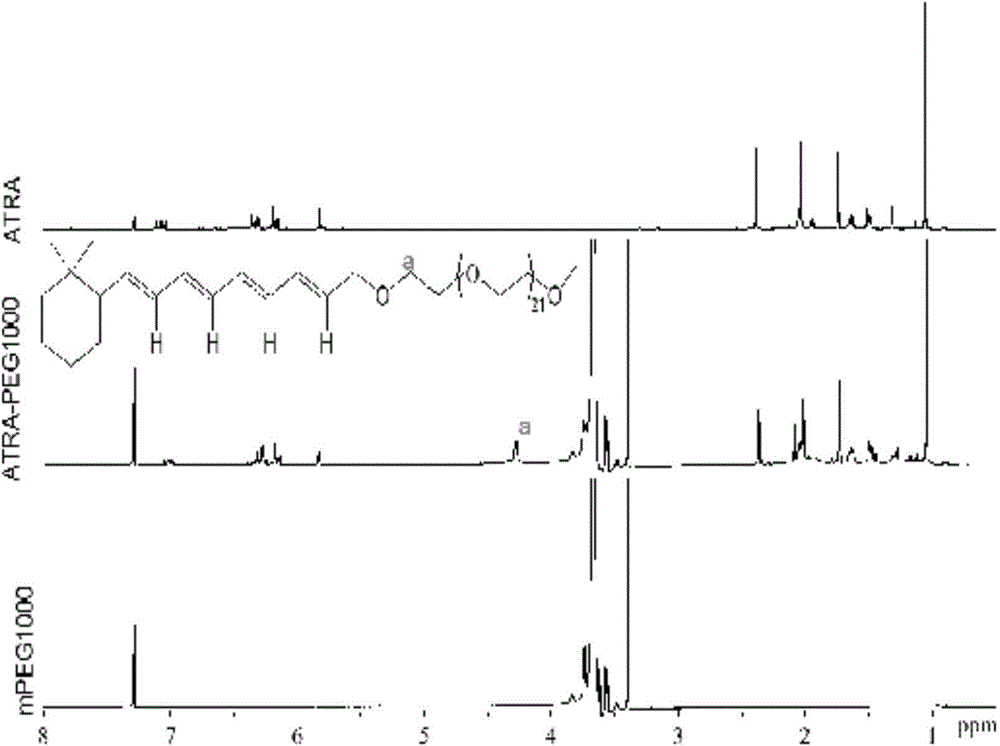
[0046] Determination by NMR 1 HNMR hydrogen spectrum to determine the structure of the block copolymer, the selected solvent is CHCl 3 , the result is as Figure 2 to Figure 5. The mass spectrum of ATRA-PEG500, the proton peak is from 1.1 to 2.3ppm, 5.8 to 7.1ppm is the characteristic peak of retinoic acid, the proton peak between 3.52-3.75ppm is H in PEG, and the proton peak at 3.37-3.38ppm is PEG The terminal methyl group (-CH 3 ) characteristic peaks, that is, mPEG proton peaks from 3.3 to 3.8ppm, and the new peak (a) about 4.2ppm belongs to methylene ( figure 2 ) near the ester bond.
Embodiment 3
[0048] Adopt infrared spectrum to determine the structure of block copolymer in embodiment 2, select KBr to be blank auxiliary material for use, the result is as follows Figure 6 to Figure 9 . ATRA-PEG500, at 3444cm -1 The broad absorption peak of hydroxyl group disappears and a new peak is about 1707cm -1 The emerging peak position is due to the conversion of the carboxy-hydroxyl group of ATRA to the ester bond in ATRA-PEG500, these results indicate the formation of PEGylated retinoic acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com