Primer and probe composition and real-time fluorescent quantitative PCR (polymerase chain reaction) kit for para-influenza virus typing detection
A real-time fluorescence quantitative and virus typing technology, applied in the field of biomedicine, can solve the problems of poor primer specificity, low accuracy of detection results, poor primer sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Design specific primers and probes for parainfluenza virus type 1 HN gene, parainfluenza virus type 2 L gene, parainfluenza virus type 3 L gene and parainfluenza virus type 4 F gene, and commission Sangon Bioengineering (Shanghai) Co., Ltd.; the nucleotide sequence information of the four sets of primers and probes obtained is shown in Table 1.
[0059] Table 1 Primer and probe composition sequence information
[0060]
[0061] Among them, the length of the HN gene amplification product of parainfluenza virus type 1 is 90 bp, the length of the amplification product of parainfluenza virus type 2 L gene is 137 bp, the length of the amplification product of parainfluenza virus type 3 L gene is 128 bp, and the length of the amplification product of parainfluenza virus type 4 F The gene amplification product is 90bp in length.
[0062] The multiplex RT-qPCR kit for the detection of parainfluenza virus typing is prepared by using the primer probe composition shown in Tabl...
Embodiment 2
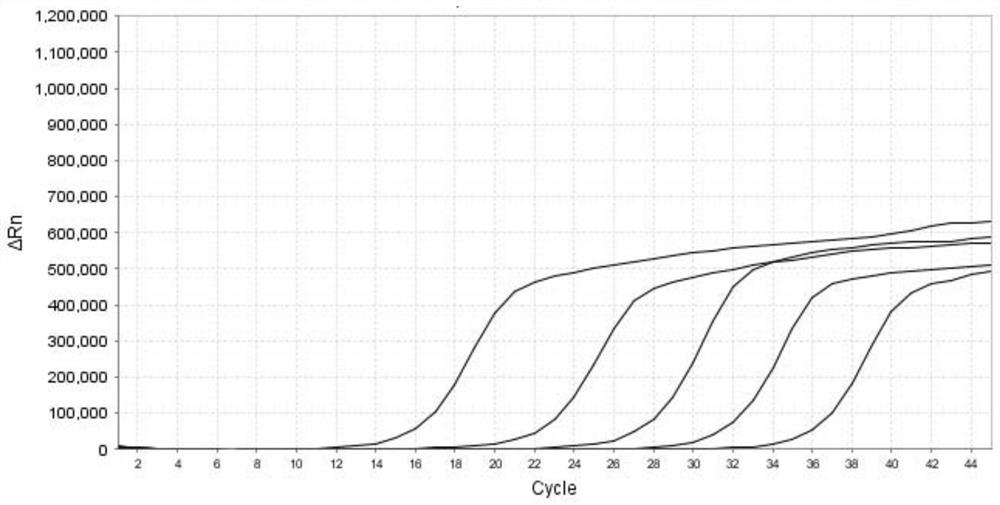
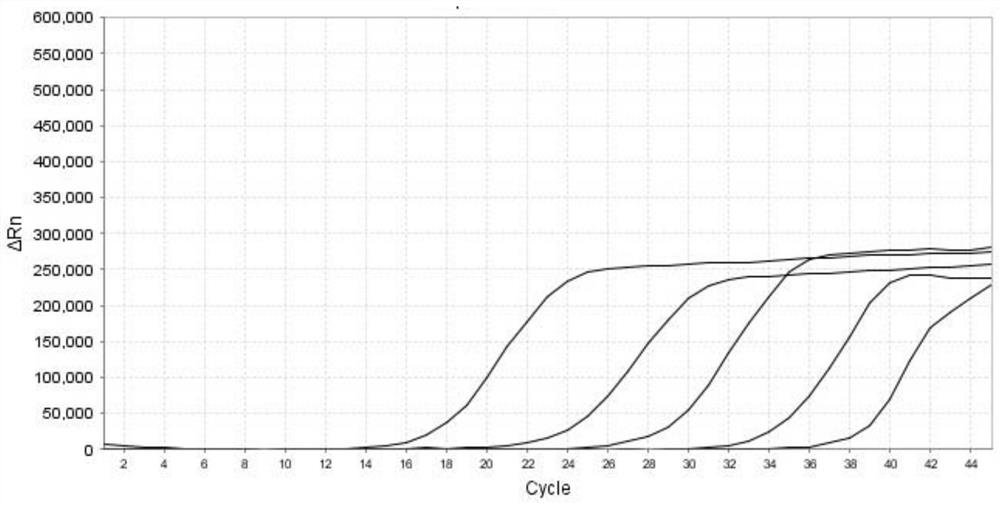
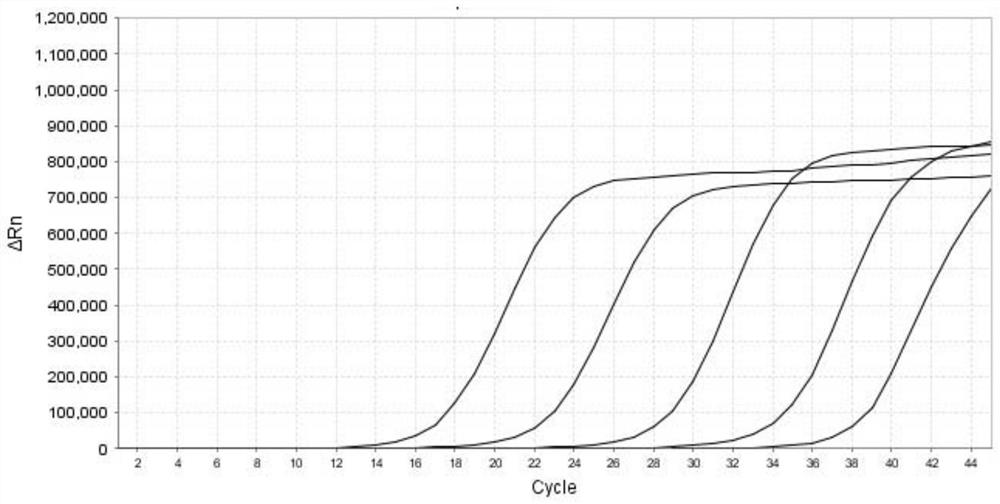
[0082] Each positive plasmid comprising the HN gene of parainfluenza virus type 1, the L gene of parainfluenza virus type 2, the L gene of parainfluenza virus type 3, and the F gene of parainfluenza virus type 4 after measuring the concentration and purity is made into a 10-fold gradient dilution respectively, obtained from 2.4×10 1 ~2.4×10 6 A total of 6 dilutions of positive plasmids in copies / μL were used as standard templates. Prepare the PCR reaction system according to Table 5 in Example 1, carry out multiple RT-qPCR reactions according to the reaction program shown in Table 6, and obtain the fluorescence amplification curve (such as figure 1 , figure 2 , image 3 and Figure 4 shown), draw a standard curve and determine the lowest detection limit. Depend on figure 1 , figure 2 , image 3 and Figure 4 shown, at a concentration of 2.4 x 10 2 ~2.4×10 6 Within the range of copies / μL, the kit of the present invention amplifies the parainfluenza virus type 1 HN ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com