Thiazole azo dye and synthesis method thereof
A technology for the synthesis of thiazole azo, which is applied in the direction of azo dyes, monoazo dyes, organic dyes, etc., can solve the problems of cumbersome and complicated modification, and achieve the effects of good purity, high yield, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
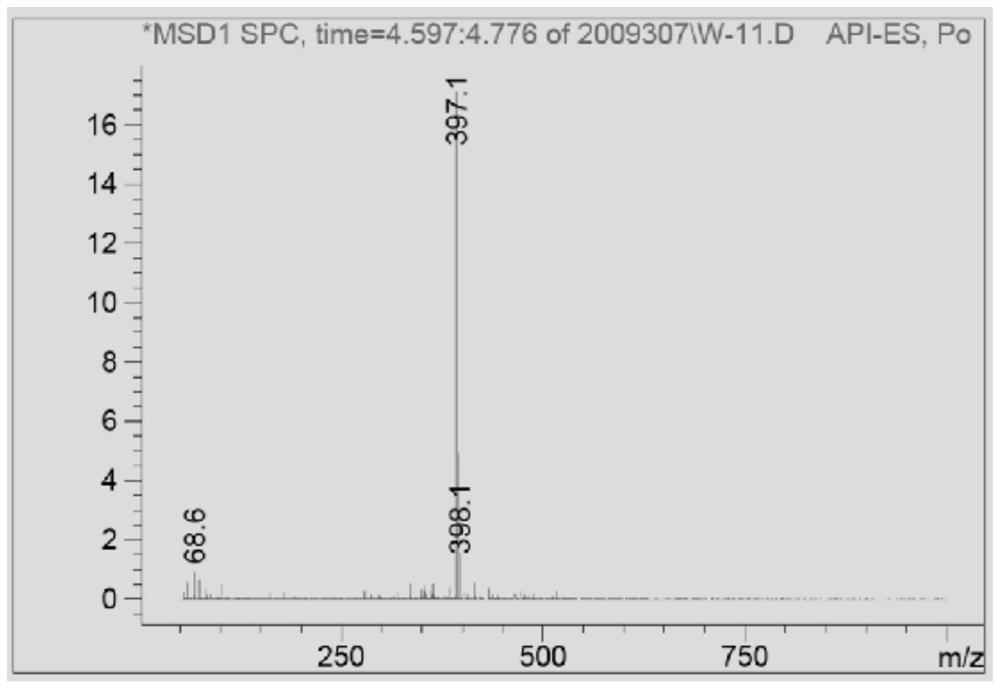
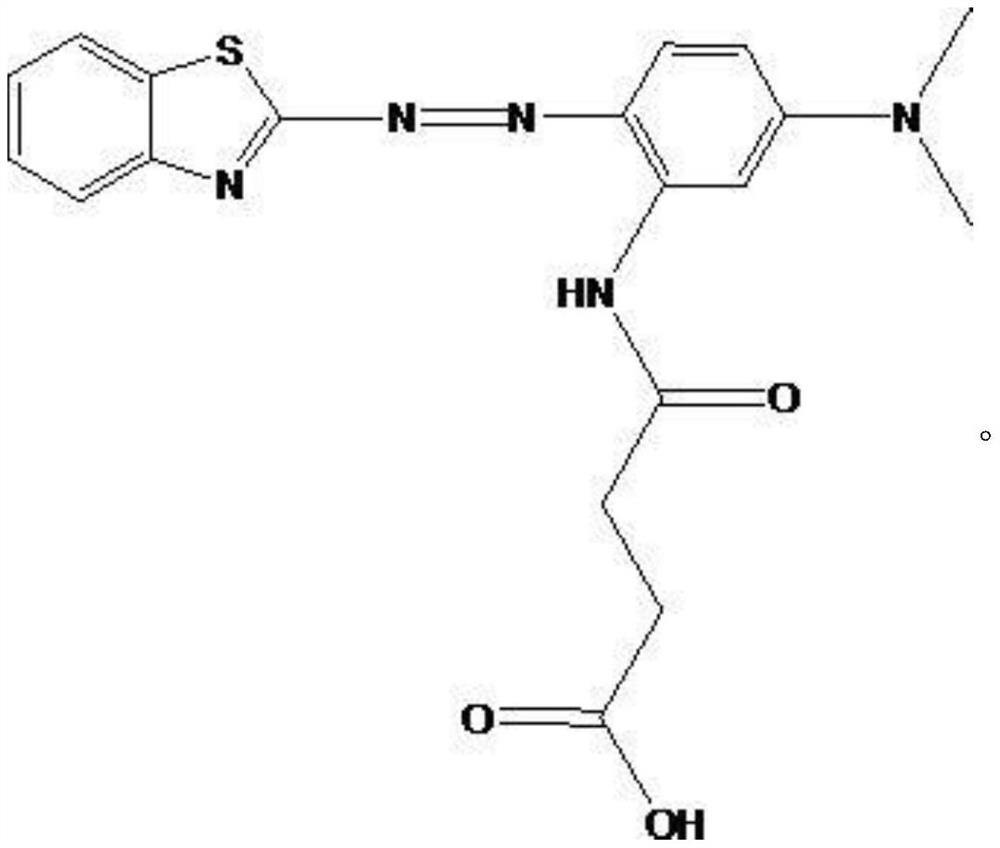
[0038] Embodiment 1, a thiazole azo dye, its chemical formula is shown in formula V.
[0039]
[0040] The synthesis method of the compound V comprises the following steps.
[0041] Acylation reaction: carry out acylation reaction of compound I and succinic anhydride in solvent I, and the reaction formula is shown in formula A.
[0042]
[0043] Formula A
[0044] The steps are as follows: Weigh 20.0 g (0.147 mol) of Compound I, dissolve it in 300 mL of acetonitrile (solvent I), add 20.0 g (0.2 mol) of succinic anhydride, and stir at 20°C until fully reacted (by TCL point Confirm that the raw material point disappears, the developer is ethyl acetate / petroleum ether volume ratio 1 / 4, carry out spot plate detection every 30min, the reaction time is 1h), then filter, keep the filter residue, after the filtrate is evaporated to dryness, the two parts of solids are Rinse with 20 mL of acetonitrile, and then dry to obtain compound II, succinic acid monoyl-(2-N,N-dimethyl)ani...
Embodiment 2~5
[0059] Examples 2-5, a thiazole azo dye, differ from Example 1 in that in the acylation reaction step, the solvent I is adjusted, as shown in Table 1 for details.
[0060] Table 1, the influence of solvent selection in the acylation reaction process
[0061]
[0062] Note: In Examples 2-5, only the solvent selection in the reaction process was adjusted, and no adjustment was made to the solution used for subsequent rinsing. Can see by above-mentioned data, in the present application, adopt acetonitrile, dichloromethane and trichloromethane as solvent, all can realize the smooth progress of reaction, have faster speed of reaction when adopting acetonitrile, may compare with the polarity of acetonitrile High related. Methanol interferes with the reaction of succinic anhydride, causing the conversion rate of compound I to be low and unable to react to the end.
Embodiment 6~15
[0063] Examples 6-15, an azothiazole dye, are different from Example 1 in that the amounts of compound I, solvent I and succinic anhydride are shown in Table 2.
[0064] Table 2, the impact of the amount of each substance in the acylation reaction on the reaction
[0065]
[0066] In the above examples, the amount of each substance used in the acylation reaction step was adjusted. It can be seen that when the concentration of compound I and succinic anhydride is lower than 0.1M, the overall reaction takes a long time, and because the obtained solution contains a lower concentration of compound II, less compound II is isolated. When the concentration of compound I and succinic anhydride is higher than 1M, because the concentration of compound I is too high, the two ends of the succinic acid group are prone to acylation in the beginning of the reaction process, resulting in more impurities, which in turn makes The yield of the final product decreases.
[0067] In addition, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com