Bridge linkers for conjugate coupling of cell-binding molecules
A linker and cell technology, applied in the field of preparing cell-binding agent-drug conjugates, can solve the problem that the DAR value is lower than 2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
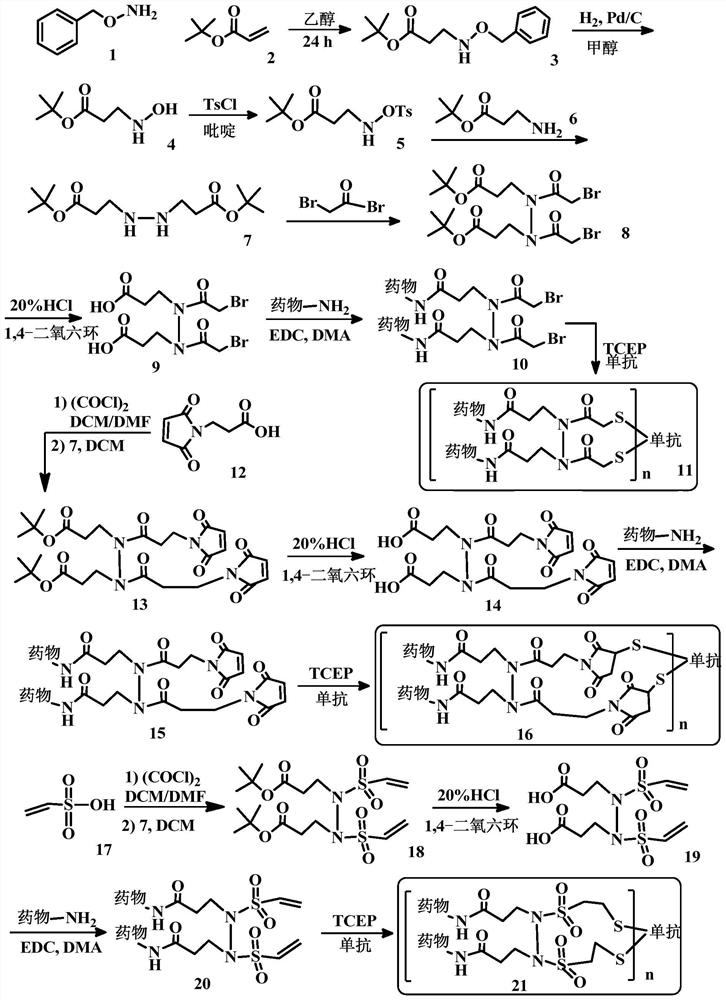
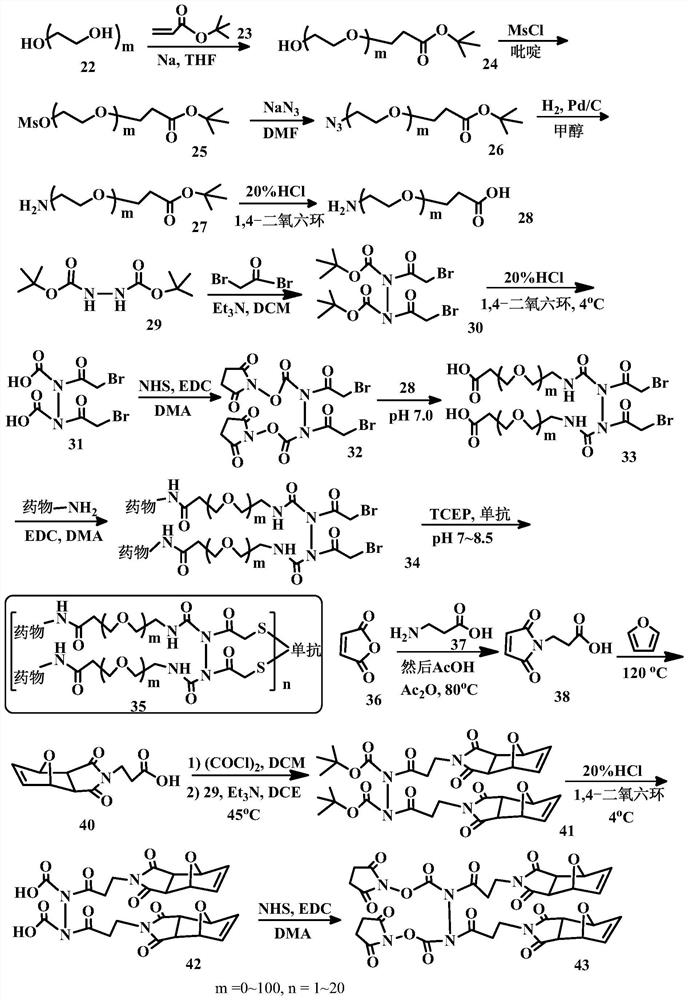
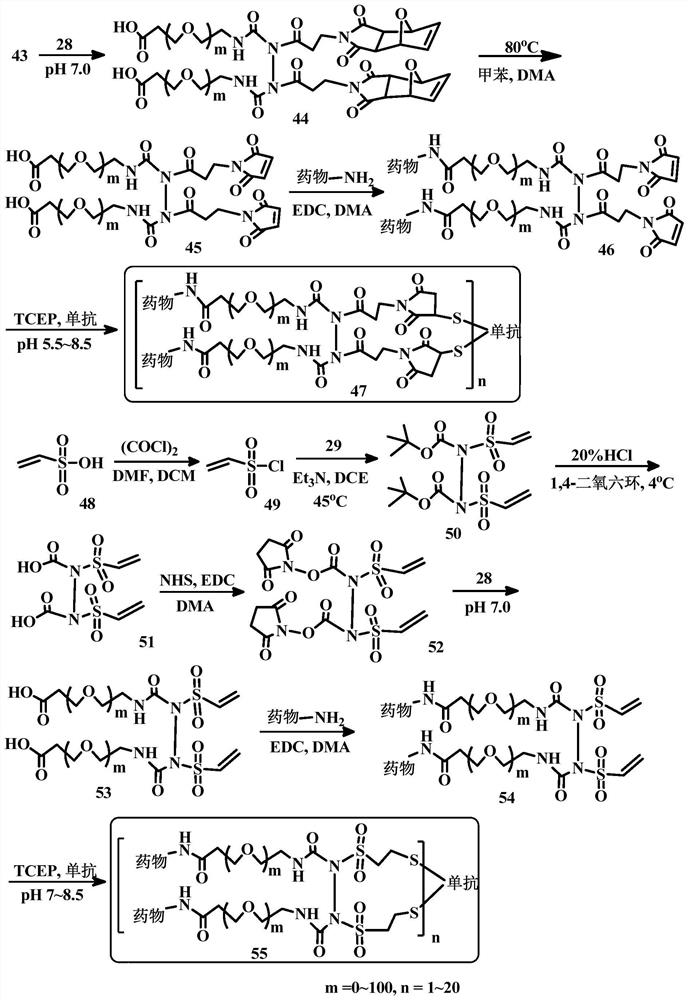
[0204] Embodiment 1. A bridging body with a structure as shown in (I), used to prepare a conjugate of a cell-binding agent and a cytotoxic agent:
[0205]
[0206] in:
[0207] Y 1 and Y 2 are the same or different functional groups that can react with a pair of sulfur atoms on the cell-binding agent to form disulfide, thioether or thioester linkages. preferred Y 1 and Y 2 For, but not limited to, N-hydroxysuccinimidyl ester, maleimide, disulfide, haloacetyl, acid halide, vinylsulfonyl, acryloyl, 2-(p-toluenesulfonyloxy) Acetyl, 2-(methylsulfonyloxy)acetyl, 2-(nitrophenoxy)acetyl, 2-(dinitrophenoxy)acetyl, 2-(fluorophenoxy)acetyl , 2-(difluorophenoxy)acetyl, 2-(pentafluorophenoxy)acetyl, 2-(trifluoromethanesulfonic acid oxy)acetyl, and / or anhydride groups, the structure of which is as follows:
[0208] N-hydroxysuccinimide ester; Maleimide group; disulfide; Haloacetyl; acid halides or esters; Vinylsulfonyl; Acryloyl; 2-(p-toluenesulfonyloxy)acetyl; 2...
Embodiment approach 2
[0213] Embodiment 2. A cell-binding agent-drug conjugate having a structure such as formula (II):
[0214]
[0215] in:
[0216] Cb is a cell binding agent, most preferably an antibody.
[0217] Drug1 and Drug2 are the same or different cytotoxic agents, which are linked by bridges in the form of alkyl, alkylene, alkenylene, alkynylene, ether, polyalkoxy, ester, amine, imine, polyamine , hydrazine, hydrazone, amide, urea, semicarbazide, carbazide, alkoxyamine, carbamate, amino acid, peptide, acyloxyamide, hydroxamic acid, disulfide bond, thioether, thioester, carbamate Ester, carbonate, heterocyclic, heteroalkyl, heteroaryl, or alkhydroxime linkages and combinations thereof are linked to the cell-binding agent.
[0218] In parentheses is the linker-drug component, which is coupled to the cell-binding agent via a pair of sulfur atoms (thiols). Sulfur atom pairs that can undergo coupling reactions are usually generated by the reduction of disulfide bonds on cell-binding agen...
Embodiment approach 3
[0220] Embodiment 3. Compounds of formula (III):
[0221]
[0222] where Cb, Z 1 ,Z 2 ,n,R 1 , R 2 , R 3 and R 4 The definition is the same as embodiment 1 and 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com