Application of anhydroicaritin in preparation of medicine for preventing and treating diseases related to platelet dysfunction
A technology for icariin and abnormal function is applied in the field of icariin in the field of preparing medicines for preventing and treating diseases related to abnormal platelet function.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
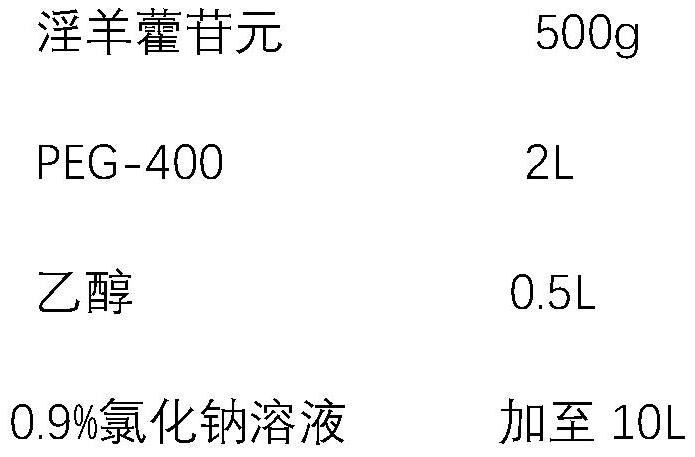
[0024] Formulation Example 1 Icariin Injection
[0025]
[0026] Preparation process: add icariin to the PEG-400 in the prescribed amount, stir to dissolve, add 0.9% sodium chloride solution to 10L, stir evenly, add 0.5% activated carbon for injection, stir, and decarbonize to get the product.
Embodiment 2
[0027] Formulation Example 2 Icariin Injection
[0028]
[0029]
[0030] Preparation process: mix the prescribed amount of ethanol and Tween-80 evenly, add icariin, stir to dissolve, add water for injection to 10L, stir evenly, add 0.5% activated carbon for injection, stir, and decarbonize, and the product is obtained.
Embodiment 3
[0031] Formulation Example 3 Icariin Injection
[0032] Icariin 1g
[0033] Ethanol 3.3L
[0034] Add water for injection to 10L
[0035] Preparation process: adding icariin in prescription amount of ethanol, stirring and dissolving, adding water for injection to 10L, stirring evenly, adding 0.5% activated carbon for injection, stirring, and decarbonizing, and the product is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com