In vivo in situ induced CAR-T cell delivery system targeting tumor and its application
A delivery system and tumor technology, applied in the field of immuno-oncology, can solve problems not related to the treatment of solid tumors, avoid complex processes and high costs, and simplify operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
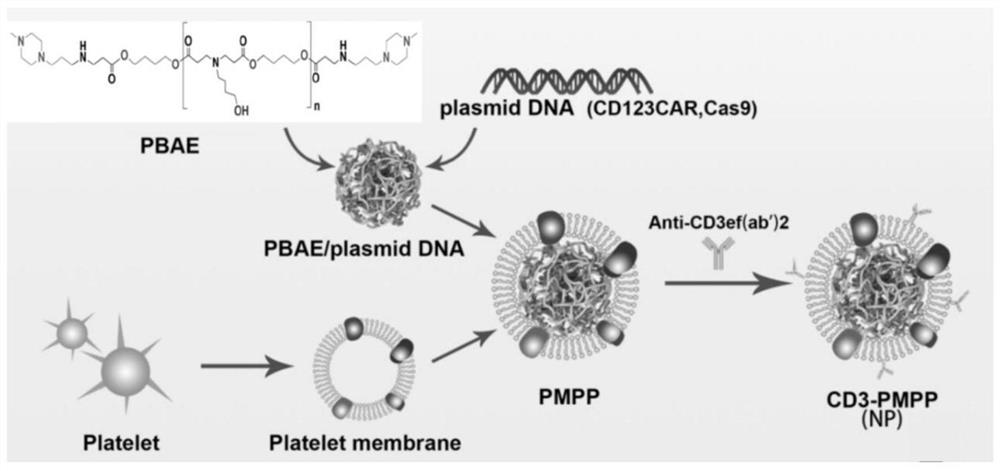
[0039] Example 1 Delivery system for in situ induction of CAR-T cells targeting tumors in vivo
[0040] 1. Preparation of PBAE / pDNA
[0041] Dilute the PBAE and CD123 gene and CRISPR plasmids (purchased by the company) in 25mM sodium acetate solution respectively, add the PBAE solution dropwise to the same volume of CRISPR plasmid solution and mix, then add the CD123 gene plasmid and mix well. Assembly to construct PBAE / pDNA nanoparticles (e.g. figure 1 shown), wherein the plasmid ratio of CD123 gene and CRISPR is 1:1, and the ratio of PBAE to CD123 gene plasmid is 30:1.
[0042] 2. Preparation of platelet membrane-encapsulated PBAE / pDNA nanocarriers
[0043] After C57BL / 6J mice were anesthetized, blood was collected from the living heart in an anticoagulant tube, platelet separation solution was added, centrifuged at 300 g for 15 min, the first plasma layer was taken into a sterile centrifuge tube, and the same volume of sample diluent was added. , centrifuged at 500g for ...
Embodiment 2
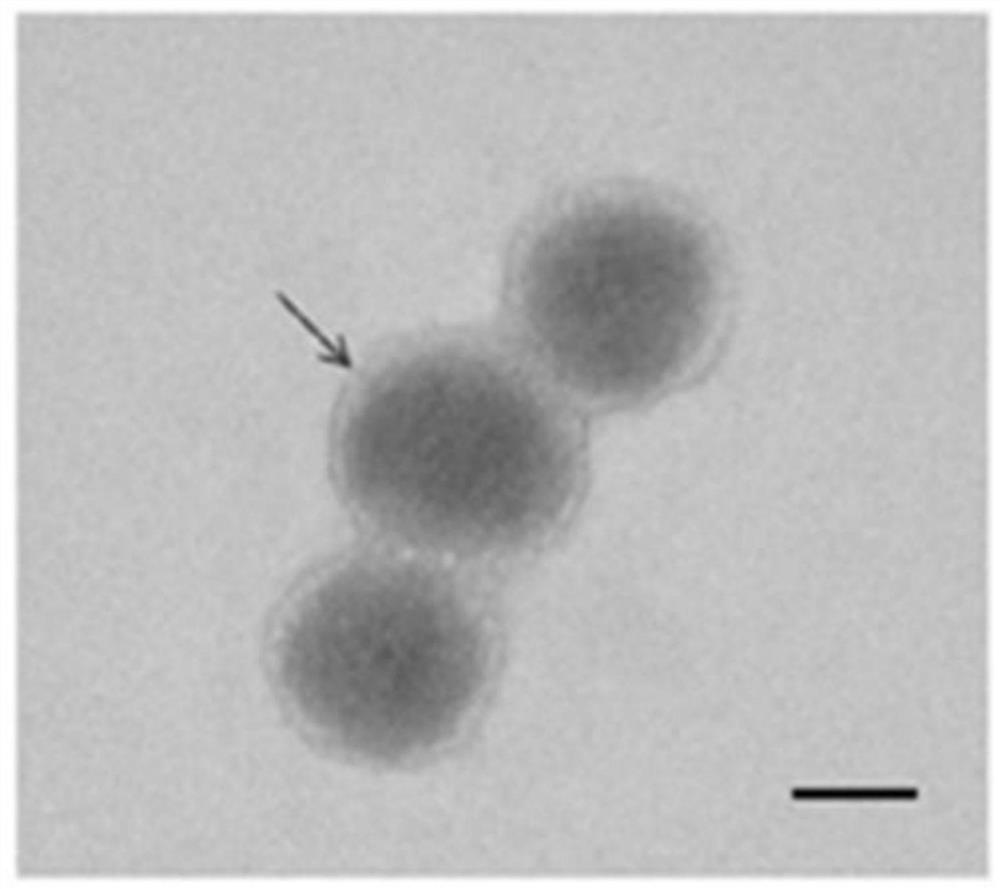
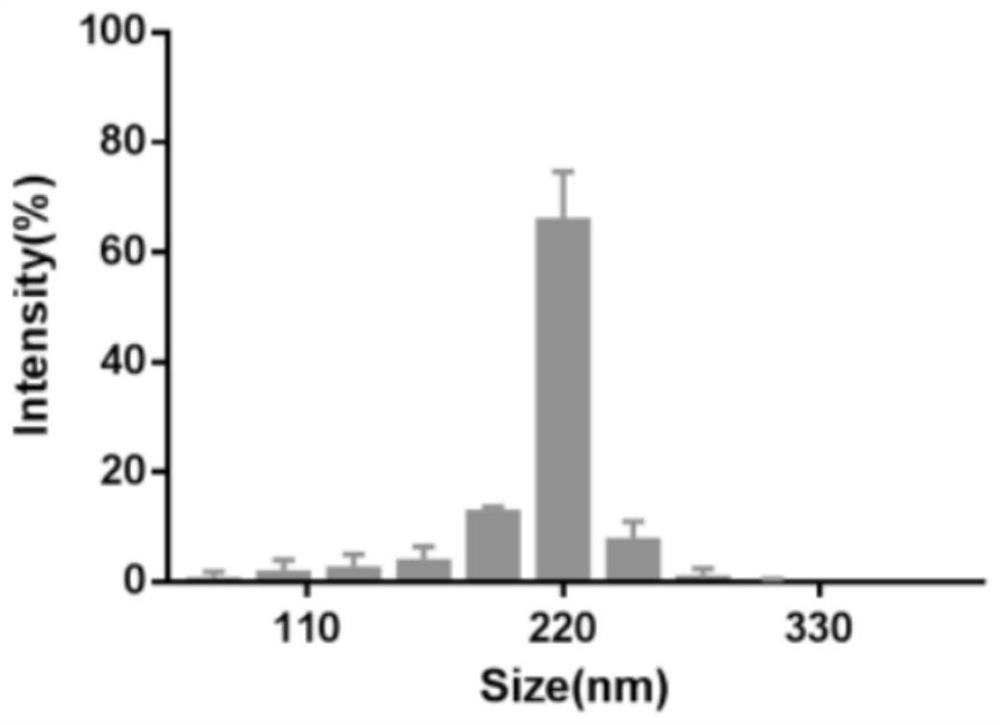
[0045] Example 2 Characterization and identification of nanoparticles
[0046] The morphology of the nanocarrier prepared in Example 1 was observed by transmission electron microscope, 5ul of the nanocarrier was diluted with deionized water 3 times, and then a small amount of the diluted sample was drawn and added dropwise to the copper mesh. The results are as follows: figure 2 As shown, the particle size of nanometers is 150-200 nm.
[0047] The size and zeta potential of nanoparticles were measured by dynamic light scattering laser nanoparticle size analyzer, and 1 mL of nanocarrier samples were taken into the particle size analyzer sample cell and the potential measurement sample cell, respectively. The results are as follows image 3 and Figure 4 As shown, the main particle size distribution of the nanoparticles is at 220 nm, and the zeta potential of the final encapsulated nanoparticle is -18 mV.
Embodiment 3
[0048] Example 3 Efficiency detection of nanoparticle transfection of plasmid into T cells in vitro
[0049] Pure pDNA, PBAE / pDNA and platelet membrane-encapsulated PBAE / pDNA groups were cultured with mouse spleen-derived T cells (PBMC), and the transfection efficiency was observed by confocal microscopy at 20 and 120 min of transfection. The result is as Figure 5 As shown, the platelet membrane-encapsulated PBAE / pDNA nanocarriers have good transfection efficiency compared to the plasmid pDNA group alone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com