Culture method for simultaneously amplifying gamma delta T and NK
A medium and co-cultivation technology, applied in the field of immunomedicine, can solve the problem of inability to use allogeneic reinfusion, achieve excellent anti-tumor effect, save time and cost of preparation, and reduce GVHD.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation of Artificial Antigen Presenting Cells (aAPC)
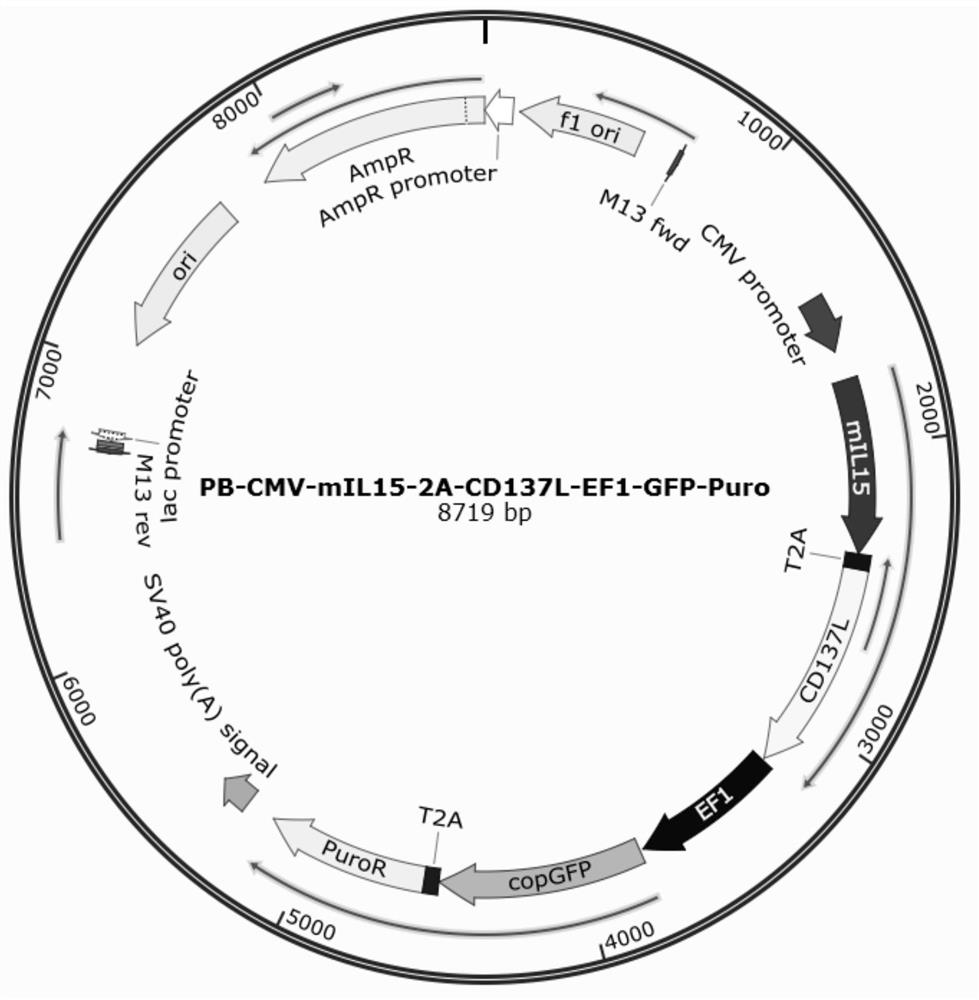
[0055] 1. Construction of PB-mIL15-CD137L-Puro vector: Membrane-type IL15 (mIL15) is connected to CD137L through a self-cleaving 2ALinker. The 2A Linker expresses 20 amino acids and can self-cleavage at the 19+1 site, wherein mIL15 The first 19 amino acids remain. Its structure is CMV-mIL15-2A-CD137L-EF1-GFP-Puro, the vector contains GFP green fluorescent protein, which is used to indicate the integration of mIL15 and CD137L genes in the cell genome; the included puromycin (Puro) resistance as drug screening markers. The mIL15-CD137L sequence was inserted between BamHI and NotI. The obtained carrier map is as figure 1 mentioned.
[0056] 2. Among them, mIL15 (membrane IL15) is anchored on the surface of K562 cell membrane through the hinge region and transmembrane region, and exerts its function by combining with IL15R on T cells. Its structure is as figure 2 shown.
[0057] 3. The CD137L mol...
Embodiment 2
[0063] Example 2: Culture of γδT and NK Mixed Cells (NK / γδT)
[0064] 1. Separation of PBMCs: Collect 50ml of peripheral blood, and add 15ml of lymphocyte separation solution to two 50ml sterilized centrifuge tubes under the normal working condition of the ultra-clean workbench. Slowly inject the peripheral whole blood into the supernatant of the lymphocyte separation liquid in 2 centrifuge tubes, and add 25-30ml of peripheral blood to each centrifuge tube. Centrifuge at 700g×20 minutes, increase speed 1, decrease speed 2, room temperature. After centrifugation, blood is separated into 4 layers consisting of plasma (upper layer), mononuclear cells between plasma and separation fluid (layer 2), separation fluid (layer 3), and erythrocyte layer (bottom layer). Collect the layer 2 mononuclear cells into a new centrifuge tube with a pipette. Add 20ml of PBS to dilute the cell suspension, and centrifuge at 500g for 10min.
[0065] 2. Stimulate γδT and NK cells on D0: remove the ...
Embodiment 3
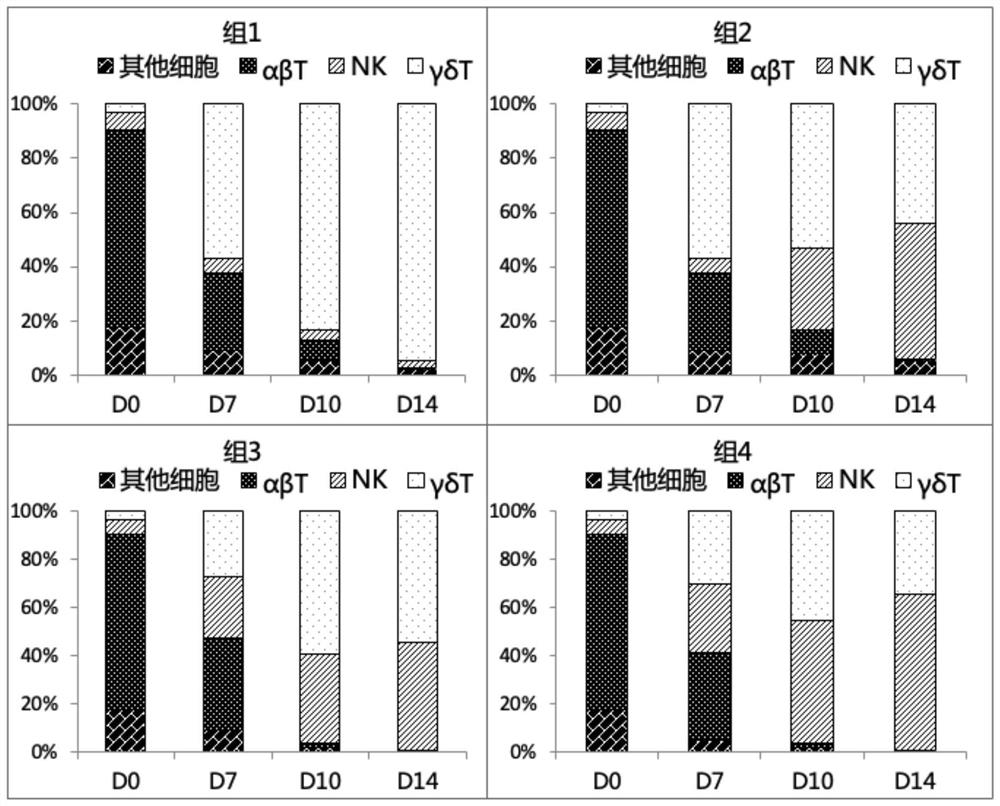
[0069] Embodiment 3: γδT and NK ratio detection
[0070] Samples were taken on Day0, Day7, Day10, and Day14 of the culture, stained with anti-human CD3 antibody, anti-human CD56 antibody, anti-human TCRαβ antibody, anti-human TCRγδ antibody, anti-human TCRVδ1 antibody, and anti-human TCRVδ2 antibody, and flow cytometric detection of NK cells and γδT cell expression.
[0071] The results are shown in Table 2 and image 3 As shown, when the cells in group 1 were expanded to 14 days (D14), the main cell subpopulation was γδT cells, accounting for 94.2%; groups 2, 3, and 4 could effectively expand γδT and NK cell subpopulations simultaneously, About 40-65%; Group 2 and Group 3 can obtain relatively balanced γδT and NK subgroups, and Group 4 has more NK ratio. γδT and NK ratios can stimulate different ratios of cell subtypes by adjusting the number of aAPCs. In addition, the four culture schemes only contain a very low proportion of αβT cells (less than 1%), and are all suitable...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com