ANTI-CD3 humanized antibody
A humanized antibody, VH-CDR3 technology, applied in the field of biomedicine, can solve the problems of limitations, uncertain efficacy, short half-life of bispecific antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0200] Antibody preparation
[0201] Any method suitable for producing monoclonal antibodies can be used to produce anti-CD3 antibodies of the invention. For example, animals can be immunized with linked or naturally occurring CD3 homodimers or fragments thereof. Suitable immunization methods may be used, including adjuvants, immunostimulants, repeated booster immunizations, one or more routes may be used.
[0202] Any suitable form of CD3 can be used as an immunogen (antigen) for producing non-human antibodies specific to CD3 and screening the biological activity of the antibodies. The priming immunogen can be full-length mature human CD3, including native homodimers, or single / multiple epitope-containing peptides. Immunogens can be used alone or in combination with one or more immunogenicity enhancers known in the art. Immunogens can be purified from natural sources, or produced in genetically modified cells. The DNA encoding the immunogen can be genomic or non-genomic i...
Embodiment 1
[0243] Example 1 Production of Humanized CD3 Monoclonal Antibody
[0244] Balb / C mice were immunized with recombinant human CD3E protein (C00E, Novoprotein). After the immunization, B cells were taken to construct a phage display library, and candidate antibodies were obtained through screening. A series of humanized antibodies were obtained by structural simulation and rational design. The VH and VL of the humanized sequence are connected by a (G4S)3 linker, and a 6HIS tag is added to the C-terminus to construct a single-chain antibody (scFv), which is expressed in E. coli and purified by a nickel column to obtain a single-chain antibody protein. The obtained humanized antibody heavy chain variable region (VH), light chain variable region (VL) sequence and CDR, such as the obtained humanized antibody heavy chain variable region (VH) sequence are as follows:
[0245] VH-CDR1 of humanized CD3 antibody (SEQ ID NO.1)
[0246] GFTFNKYA
[0247] VH-CDR2 of humanized CD3 antibody ...
Embodiment 2
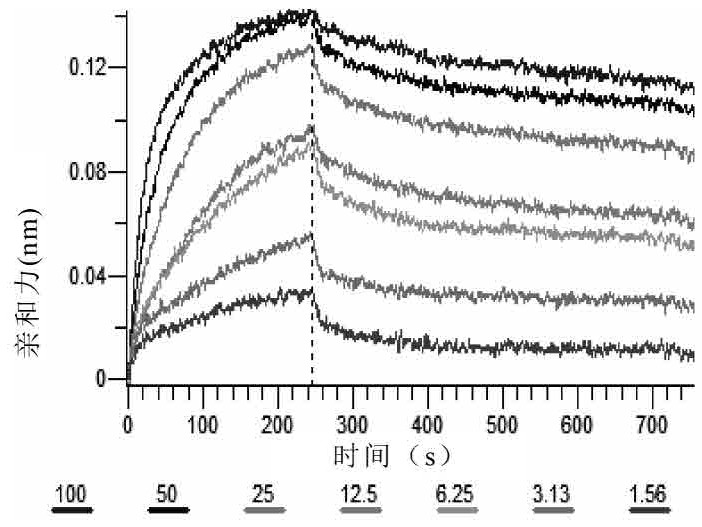
[0261] Example 2 Affinity Detection of CD3 Antibody
[0262] This example mainly describes the detection of affinity between CD3 antibody in the form of single chain antibody and human and monkey recombinant CD3E protein.
[0263] Affinity with human CD3 protein
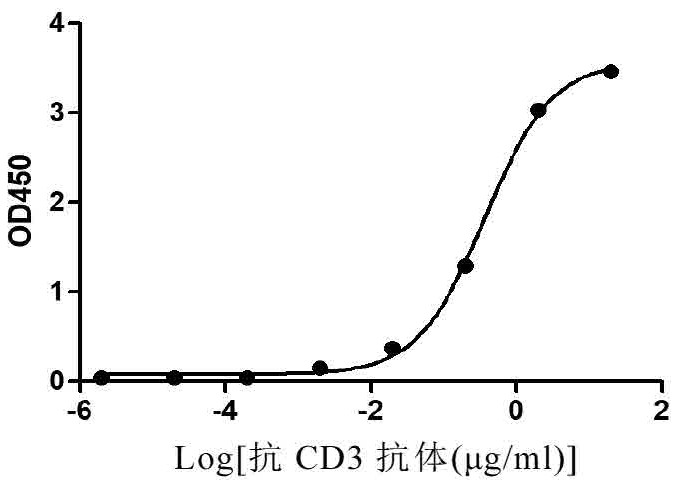
[0264] The affinity between CD3 antibody and recombinant hCD3 protein (CP19, Novoprotein) was determined by ELISA, the recombinant hCD3 was coated on the plate, and the CD3 antibody was diluted 10 times (starting from 20μg / ml), see figure 1 .
[0265] The calculated EC50 was 0.3642 μg / ml.
[0266] Affinity with monkey CD3 protein
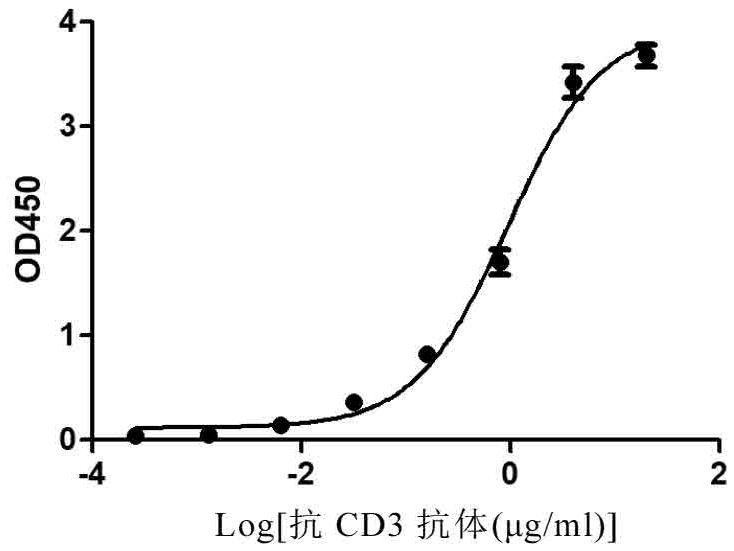
[0267] The affinity between CD3 antibody and recombinant monkey CD3 protein (CW07, Novoprotein) was determined by ELISA, the recombinant monkey CD3 was coated on the plate, and the CD3 antibody was diluted 10 times (starting from 20μg / ml), see figure 2 .
[0268] The calculated EC50 was 0.9381 μg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com