Combination therapy for treatment of hematological diseases
A technology for blood diseases and leukemia, which is applied in the field of combination therapy for blood diseases, and can solve problems such as incurable diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0166] Example A: In Vitro JAK Kinase Assay
[0167] Selective JAK1 inhibitors that can be used in combination with immunomodulators and steroids for the treatment of hematological diseases or disorders were tested for their inhibitory activity on JAK targets according to the following in vitro assay described in Park et al., Analytical Biochemistry 1999, 269, 94-104 . The catalytic domains of human JAK1 (a.a.837-1142), JAK2 (a.a.828-1132) and JAK3 (a.a.781-1124) with N-terminal His tags were expressed in insect cells using baculovirus and purified. The catalytic activity of JAK1, JAK2 or JAK3 was determined by measuring the phosphorylation of biotinylated peptides. Phosphorylated peptides were detected by homogeneous time-resolved fluorescence (HTRF). ICs of compounds were measured for each kinase in 40 μL reactions 50 , the reaction contained enzyme, ATP and 500 nM peptide in 50 mM Tris (pH 7.8) buffer with 100 mM NaCl, 5 mM DTT and 0.1 mg / mL (0.01%) BSA. For 1mM IC 50T...
Embodiment 1
[0168] Example 1: Clinical research on the treatment of multiple myeloma with JAK1 selective inhibitors
[0169] I. Design:
[0170] ·Single group
[0171] 2 stages
[0172] Triple combination: itatinib (compound 1, Table 1), steroid (methylprednisolone or dexamethasone), and lenalidomide
[0173] II. Main objectives:
[0174] • ORR (CR+VGPR+PR). ORR (Objective Response Rate) is defined as after each 4-week or 28-day treatment cycle, partial response (PR), very good Percentage of participants who responded (VGPR) or completely responded (CR).
[0175] III. Primary endpoint
[0176] ·IMWG standard tools
[0177] IV. Secondary endpoints:
[0178] · Overall survival (OS)
[0179] · Progression-free survival (PFR)
[0180] Time to Response (TTR): defined as the time from initiation of therapy to first sign of PR, VGPR, or CR
[0181] Duration of Response (DOR): Measured from when the responder starts responding to when the responder loses response
[0182] The safety a...
Embodiment 2
[0201] Example 2. In Vitro Analysis of the Viability of Multiple Myeloma Cell Lines by Itatinib, Lenalidomide, and Dexamethasone Combination Therapy
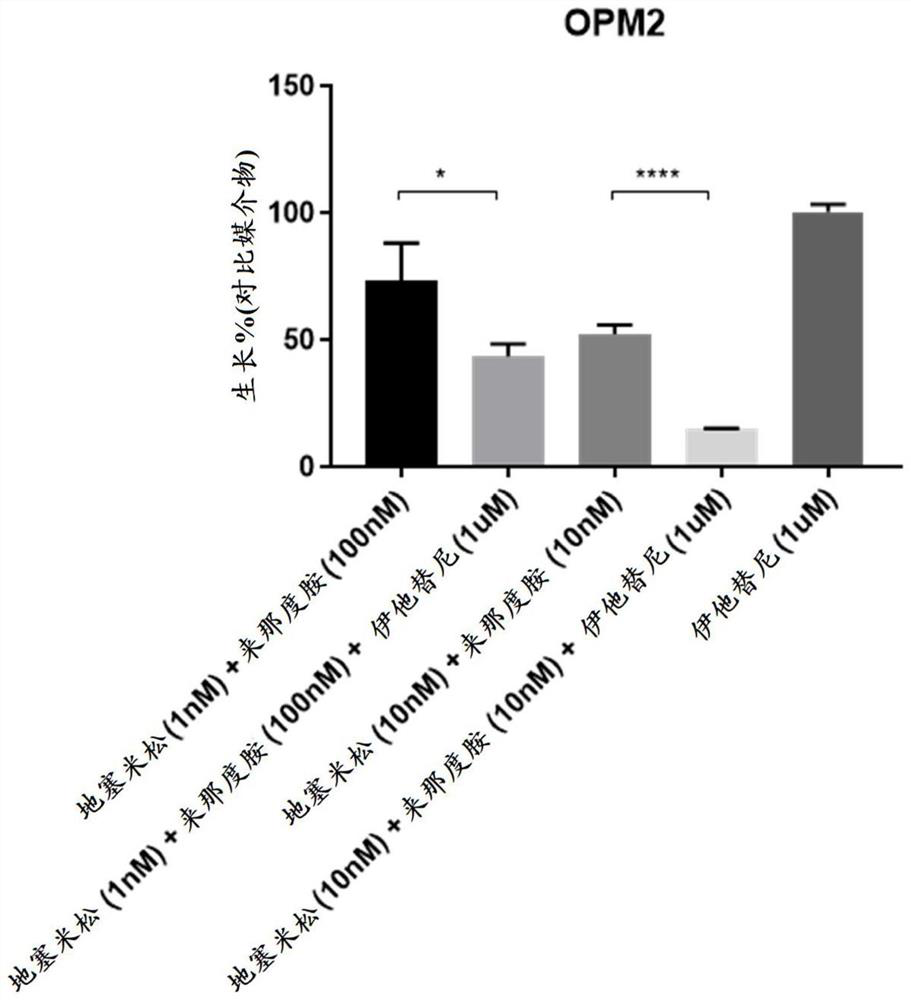
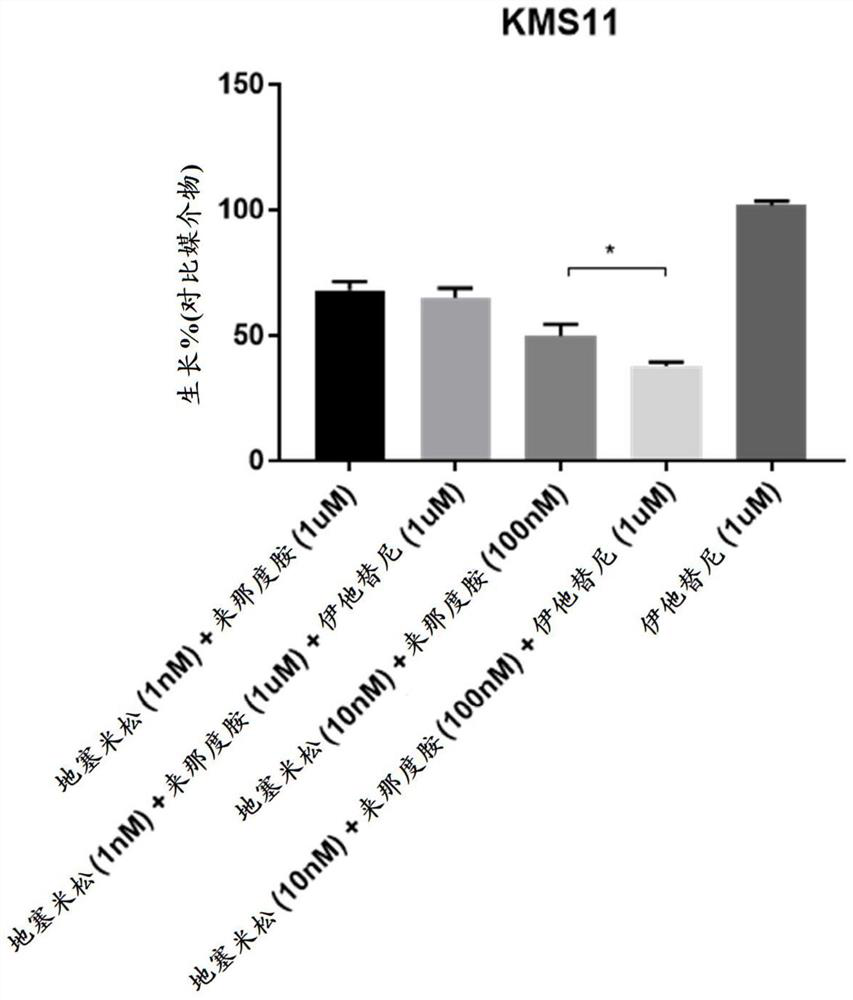
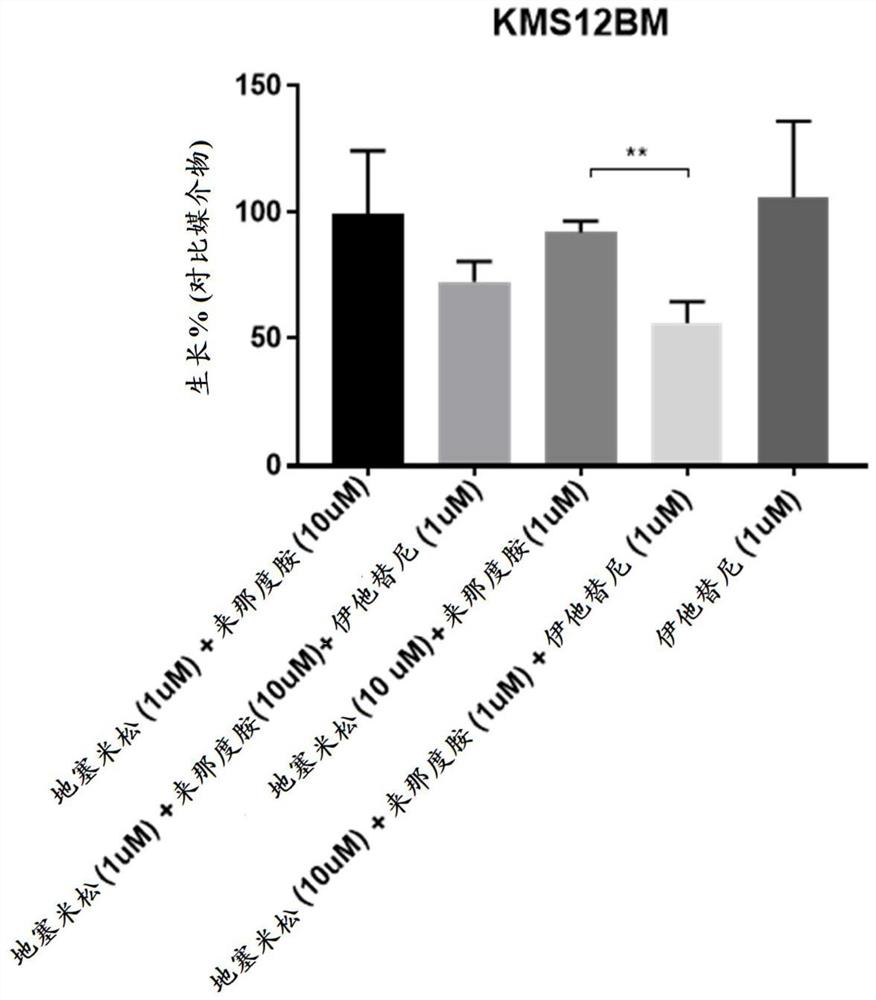
[0202] Human multiple myeloma cell lines KMS12BM, OPM2 (DSMZ), MM1.R, MM1.S (ATCC) and KMS11 (JCRB) were mixed in 100 μL medium with 10 4 Cells were seeded into white 96-well plates (Greiner Bio One). A combination of dexamethasone (Sigma), lenalidomide (Chemscene), itatinib or DMSO control was then added. Doses were chosen based on preliminary studies aimed at finding the sensitivity of each cell line to these agents. Each dose combination was performed in triplicate. After 72 hours, Cell Titer Glo (Promega) assay was performed according to the manufacturer's protocol to assess cell viability.
[0203] Such as Figure 1-Figure 5 As shown, none of the cell lines tested were sensitive to itatinib as a single agent as evidenced by no change in viability when compared to DMSO-treated controls. Each cell line has a different sen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com