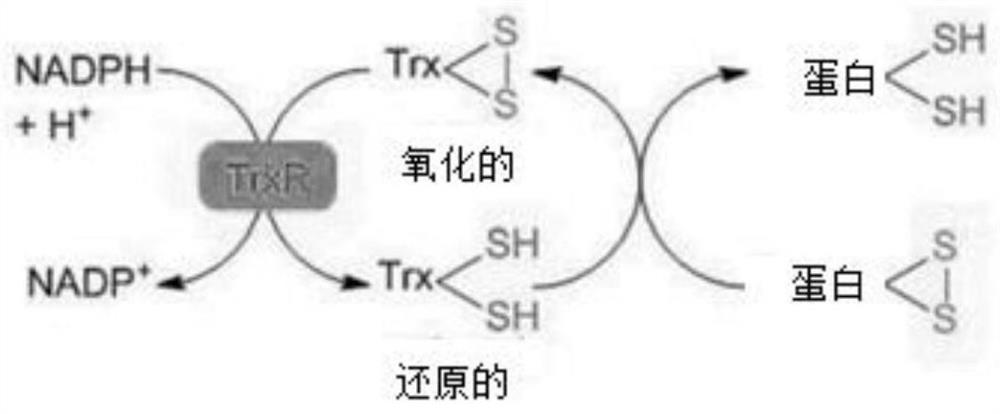
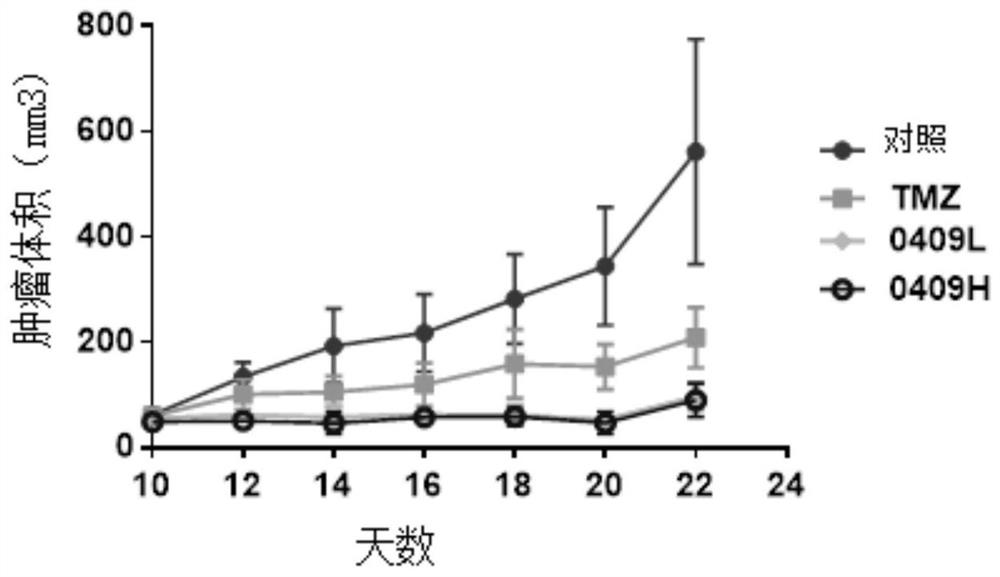
Tetrazine substituent group-containing aromatic isoselenazole compound as well as synthesis method and application thereof
A compound and substituent technology, applied in the synthesis of aryl isoselenazole compounds, the application field of medicine, can solve the problems of peripheral neurotoxicity, bone marrow suppression, side effects, etc., and achieve good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] 3,4-Dihydro-3-methyl-4-oxo-N-(3(2H)-oxo-1,2-benzisoselazol-2-yl)-ethyl-imidazo[5 ,1-d]-1,2,3,5-tetrazine-8-carboxamide (No. 1)
[0097] 1) Add 28g of 2-aminobenzoic acid to a beaker, and add a mixture of 40mL of concentrated hydrochloric acid and 40mL of water, bathe in ice, keep the reaction temperature below 5°C, and slowly add NaNO dropwise 2 (18g dissolved in 40mL water), continue to react for 2 hours after dripping to obtain a diazonium salt solution, and set aside; take 100mL water and add 16g NaOH to it, place it at 50°C and stir to dissolve, then add Liandi Sodium sulfite 17.6g, add 16g selenium powder after clarification, continue to react for 3h after adding, make Na 2 Se 2 solution, set aside; under stirring conditions, the diazonium salt was added dropwise to Na 2 Se 2 In the solution, keep the reaction temperature below 5°C, and continue to react for 2 hours after the dripping, and ensure that the solution is alkaline. After the reaction was completed,...
Embodiment 2
[0105] 3,4-Dihydro-3-methyl-4-oxo-N-(3(2H)-oxo-5-fluoro-1,2-benzisoselenazol-2-yl)-ethyl- Imidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxamide (Code 2)
[0106] 1) Add 7.75g of 2-amino-5-fluorobenzoic acid to a beaker, and add a mixture of 15mL of concentrated hydrochloric acid and 15mL of water, in an ice bath, keep the reaction temperature below 5°C, and slowly add NaNO dropwise 2 (4.35g dissolved in 10mL water), continue to react for 2 hours after dripping, and prepare a diazonium salt solution for later use; take 30mL water and add 4g NaOH to it, place it at 50°C and stir to dissolve, then add a small amount of continuous Sodium disulfite 4.35g, after clarification, add 4g of selenium powder, continue to react for 2-3h after adding, and obtain Na 2 Se 2 solution, set aside; under stirring conditions, the diazonium salt was added dropwise to Na 2 Se 2 In the solution, keep the reaction temperature below 5°C, and continue to react for 2 hours after the dripping, and ensure tha...
Embodiment 3
[0114] 3,4-Dihydro-3-methyl-4-oxo-N-(3(2H)-oxo-6-fluoro-1,2-benzisoselenazol-2-yl)-ethyl- Imidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxamide (Code 3)
[0115] 1) Add 7.75g of 2-amino-6-fluorobenzoic acid to a beaker, and add a mixture of 15mL of concentrated hydrochloric acid and 15mL of water, in an ice bath, keep the reaction temperature below 5°C, and slowly add NaNO dropwise 2 (4.35g dissolved in 10mL water), continue to react for 2 hours after dripping, and prepare a diazonium salt solution for later use; take 30mL water and add 4g NaOH to it, place it at 50°C and stir to dissolve, then add a small amount of continuous Sodium disulfite 4.35g, add 4g selenium powder after clarification, continue to react for 3h after adding, make Na 2 Se 2 solution, set aside; under stirring conditions, the diazonium salt was added dropwise to Na 2 Se 2 In the solution, keep the reaction temperature below 5°C, and continue to react for 2 hours after the dripping, and ensure that the soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com