Small molecule peptide with analgesic effect and specific antibody thereof
An antibody and active technology, applied in the field of small molecule peptides and their specific antibodies, can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
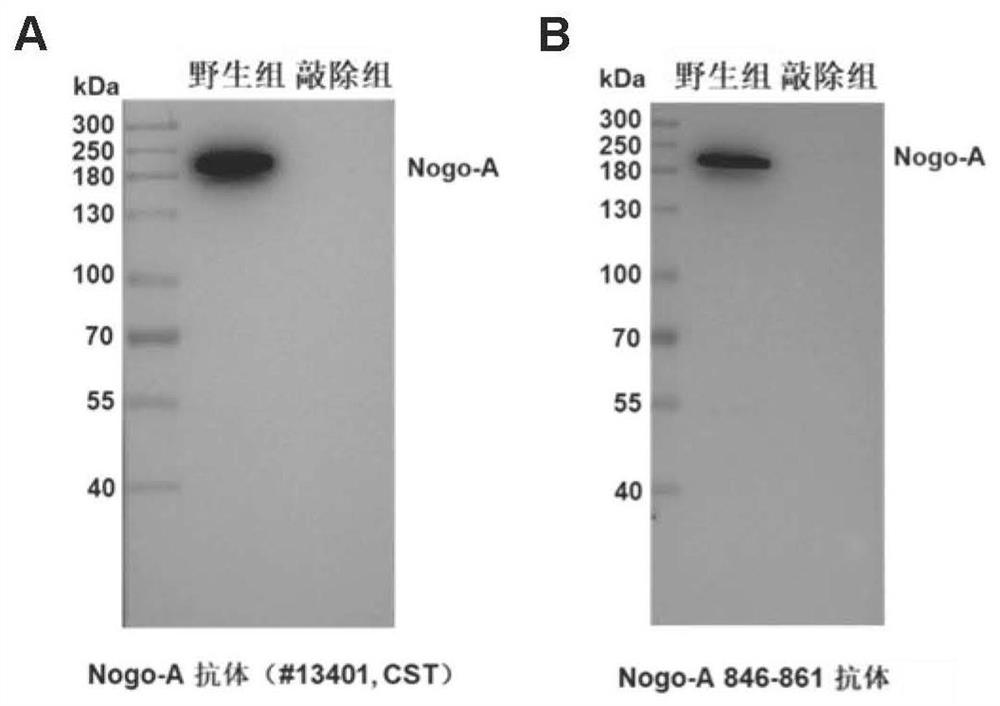
[0067] Example 1, the discovery of functional fragments—Nogo-Aaa 846-861
[0068] The human Nogo-A protein has 1192 amino acid residues (SEQ ID No.1), using the method of bioinformatics, by analyzing the structural characteristics of the human Nogo-A protein such as spatial structure, hydrophilicity, flexibility The bioinformatics analysis of the protein secondary structure, antigenicity and surface exposure probability revealed two potential new functional domains, namely Nogo-Aaa435-451 and Nogo-Aaa 872-888. At the same time, by comparing the amino acid sequences (SEQ ID No.2) of human-derived and rat-derived Nogo-A proteins, two potential rat sequences of new functional domains in bioinformatics analysis were found: Nogo-Aaa 415 -430 and Nogo-Aaa 846-861. The specific sequence of the functional fragment is shown in Table 1.
[0069] Table 1
[0070]
Embodiment 2
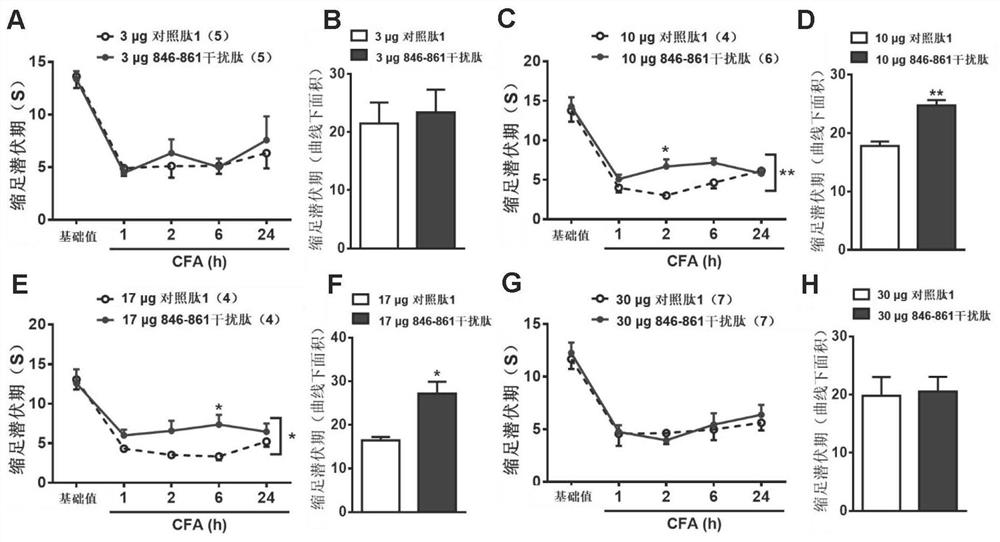
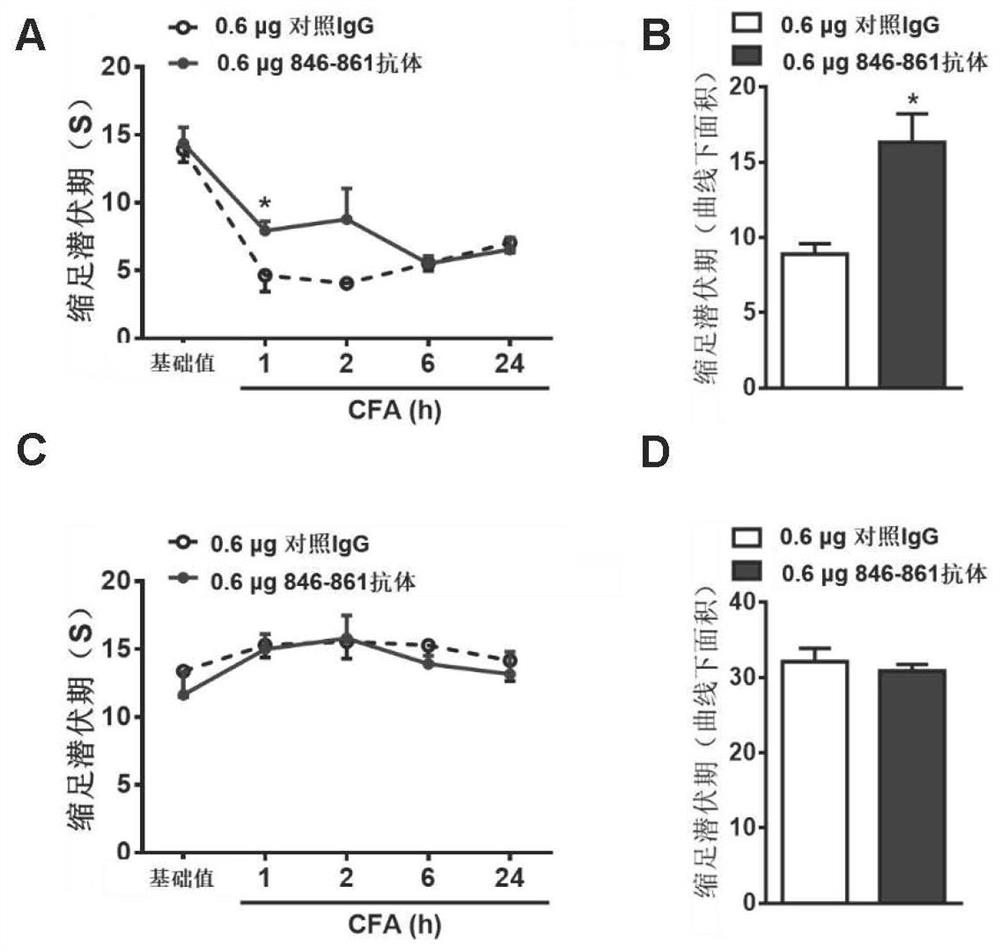
[0071] Example 2. Functional verification of Nogo-Aaa 846-861 fragment and its antagonistic peptide and specific antibody
[0072] 1. Preparation of Experiment-Related Peptides
[0073] Competitive antagonistic peptide 846-861PE of the Nogo-Aaa 846-861 fragment: Synthesized by Gil Biochemical (Shanghai) Co., Ltd., the sequence is PTFVSAKDDSPKLAKE, dissolved in sterile saline according to the purity, and the final concentration is 1 μg / μl. Scramble1 (control peptide 1): Synthesized by Jill Biochemical (Shanghai) Co., Ltd., the sequence is KDKESLDTPVAFAKS, dissolved in sterile saline according to the purity, and the final concentration is 1 μg / μl.
[0074] Nogo-Aaa 415-430 competitive antagonistic peptide 415-430PE: Synthesized by Jill Biochemical (Shanghai) Co., Ltd., the sequence is KDSEGRNEDASFPSTP, dissolved in sterile saline according to the purity, and the final concentration is 1 μg / μl. Scramble2 (control peptide 2): Synthesized by Gill Biochemical (Shanghai) Co., Ltd., ...
Embodiment 3
[0130] Example 3, Nogo-Aaa 846-861 fragments and their antagonistic peptides and specific antibodies regulate TRPV1
[0131] 1. The regulatory effect of antagonistic peptides on TRPV1
[0132] Experimental animals: adult male SD rats (Sprague-Dawley Rat, body weight 150-200 g, 6-8 weeks old).
[0133] Refer to the previous method for subarachnoid catheterization.
[0134] Rats underwent subarachnoid catheterization for 5 days to measure the basic pain threshold (except for abnormal rats). The rats were randomly divided into two groups, and then subarachnoid injections of 10 μg of competitive antagonistic peptide 846-861PE and As a control peptide Scramble 1, Western Blot method was used to detect the change of TRPV1 protein (TRPV-1, sc-12498, Santa Cruz Biotechnology) content in the basal state.
[0135] Rats underwent subarachnoid catheterization for 5 days to measure the basic pain threshold (except for abnormal rats). The rats were randomly divided into two groups, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com