Molecular chaperone expression vector and strain for improving secretory expression of phytase in pichia pastoris
A molecular chaperone and expression vector technology, applied in the field of genetic engineering, can solve the problems of insignificant, increased expression, and not significantly increased expression of mannanase, and achieves the effect of overcoming poor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The construction of embodiment 1 VectorB, VectorV, VectorH vector
[0050] 1. Construction of VectorB vector
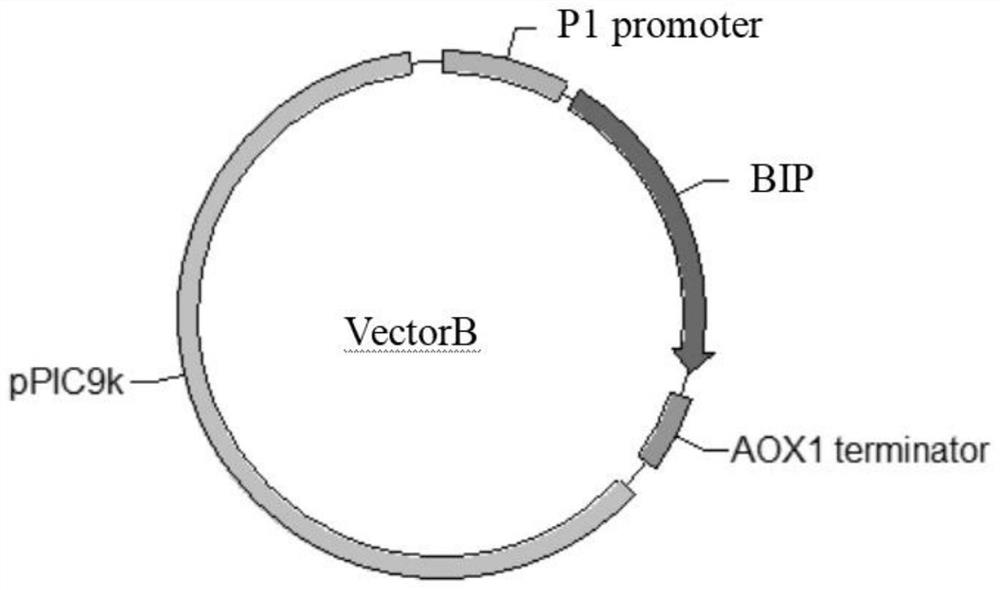
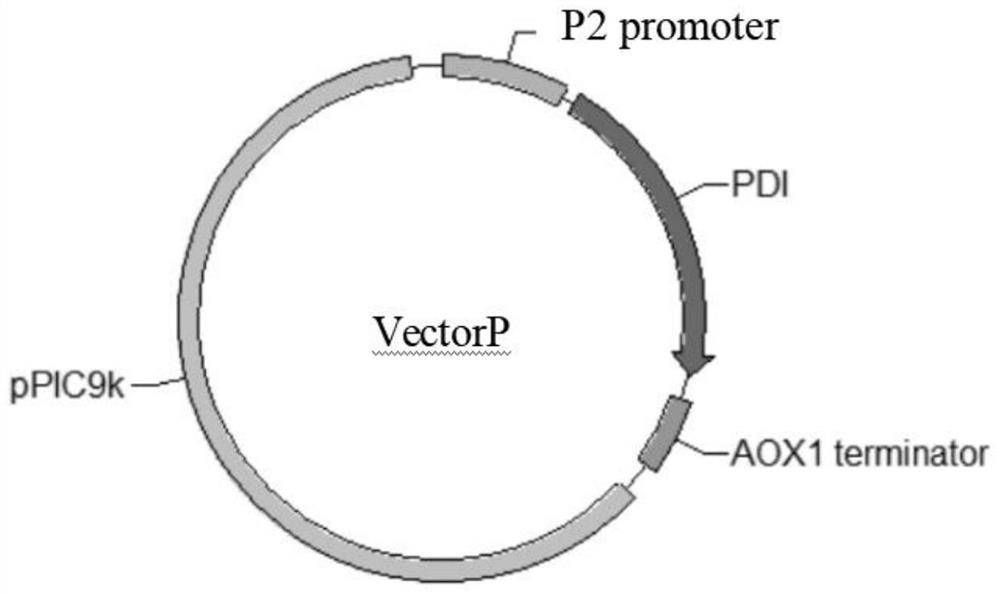
[0051] Using the Pichiapastoris X33 genome and the pPIC9k plasmid as templates, two pairs of primers were designed to amplify the two fragments of the BIP gene and the pPIC9k vector by PCR, and a fusion region was introduced into the primers. Fragment fusion, such as figure 1 As shown, a VectorB circular plasmid was constructed, and the fusion circular plasmid was transformed into E. coli Topl0 competent cells. The recombinant transformants were screened on a plate containing Amp (100 μg / mL) antibiotics, and part of the transformants were picked and transferred to LB+Amp liquid medium Incubate at 37°C for 3 hours, use the verification primers BIP-F11 and BIP-R11 to carry out bacterial liquid PCR verification, the fragment size is about 2kb, and the transformants are sent for sequencing. The construction method of VectorV and VectorH refers to VectorB, and the ...
Embodiment 2
[0093] Application of embodiment 2 vectors VectorBP, VectorVP, VectorHP in Pichia pastoris secreting and expressing thermostable phytase
[0094] (1) Expression of VectorBP, VectorVP, and VectorHP in thermostable phytase expression host V1
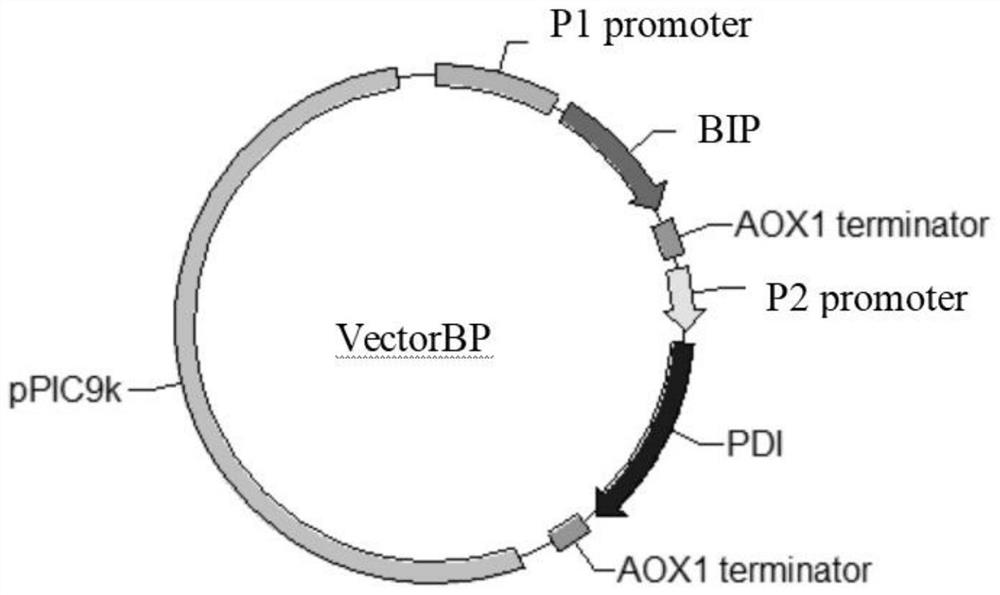
[0095] Digest VectorBP, VectorVP, VectorHP, VectorB, VectorV, VectorH, and VectorP vectors into linearized DNA fragments with restriction endonucleases, respectively purify VectorBP, VectorVP, VectorHP, VectorB, VectorV, VectorH, VectorP linearized DNA fragments with purification kits For DNA fragments, the linear DNA fragments of VectorBP, VectorVP, VectorHP, VectorB, VectorV, VectorH, and VectorP were respectively transformed into V1 competent cells containing the high-temperature-resistant phytase gene by electroporation, and V11, V12, and V13 were respectively constructed , V14, V15, V16, V17 high-temperature-resistant phytase expression strains, spread on YPD+G418 plate, culture upside down at 30°C for 3-4 days, pick about 300 single ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com