Human primary myelofibrosis cell strain and application thereof
A technology of myelofibrosis and cell lines, which is applied in the fields of biology and oncology, can solve the problems of unestablished cell lines of PMF patients, and achieve the effect of shape stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1ZY
[0027] Example 1 ZYXY-M2 cell line preparation
[0028] Primary cell culture: fresh high white blood cell isolation specimens obtained from the First Affiliated Hospital of Zhejiang University School of Medicine (male, 62 years old, PMF, white blood cell 178.1*10 9 / L) Immediate isolation of leukemic mononuclear cells. In a biosafety cabinet, take 6ml of the separation solution and add it dropwise to a 15ml sterile centrifuge tube previously added with 6ml of the lymphocyte separation solution, and centrifuge at 2000 rpm for 20 minutes. After centrifugation, take the mononuclear cell layer into a new 15ml sterile centrifuge tube, add 5ml sterile 1xPBS to resuspend the cells, and centrifuge at 2000 rpm for 5 minutes. After discarding the supernatant, add sterile erythrocyte lysate to lyse the cells at room temperature for 5 minutes, then centrifuge at 2000 rpm for 5 minutes. Discard the supernatant, add 5ml IMDM complete medium (IMDM 90% + fetal bovine serum 10%) to resuspend...
example 2
[0031] Biological properties and application of example 2 people's acute myeloid leukemia cell line
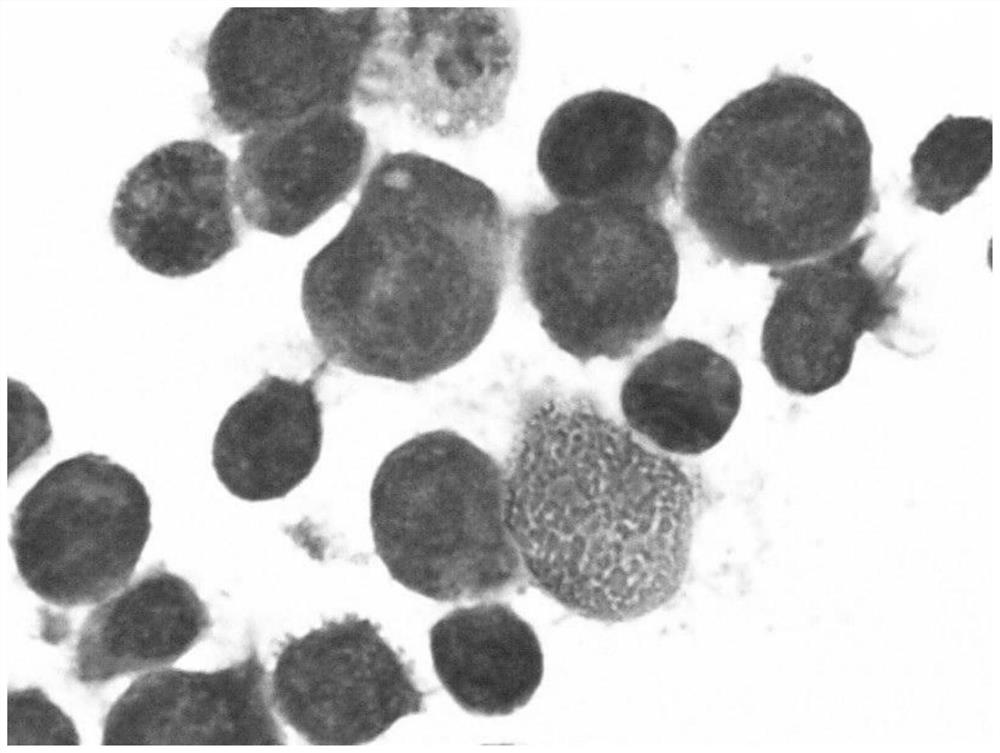
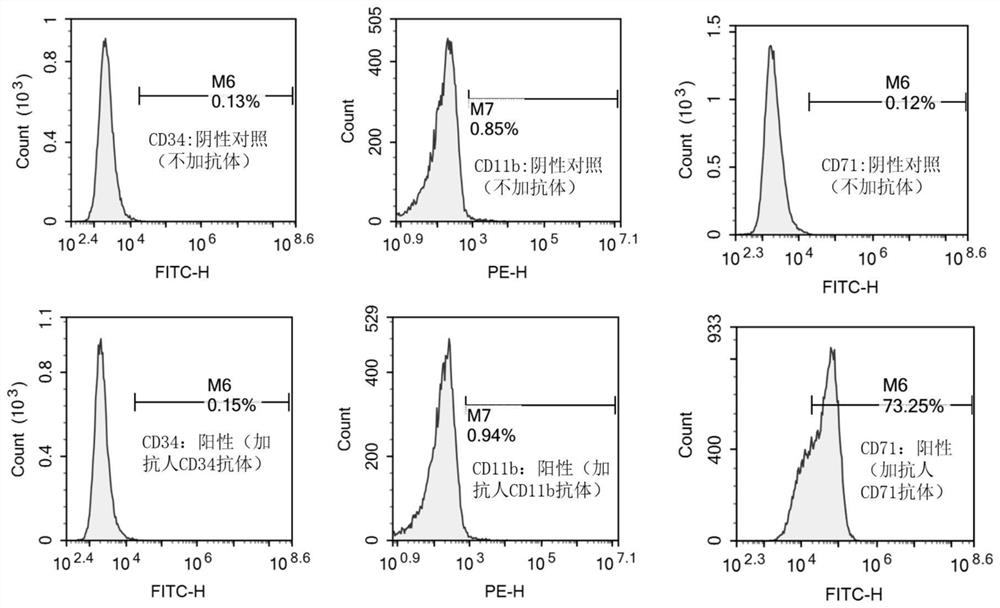
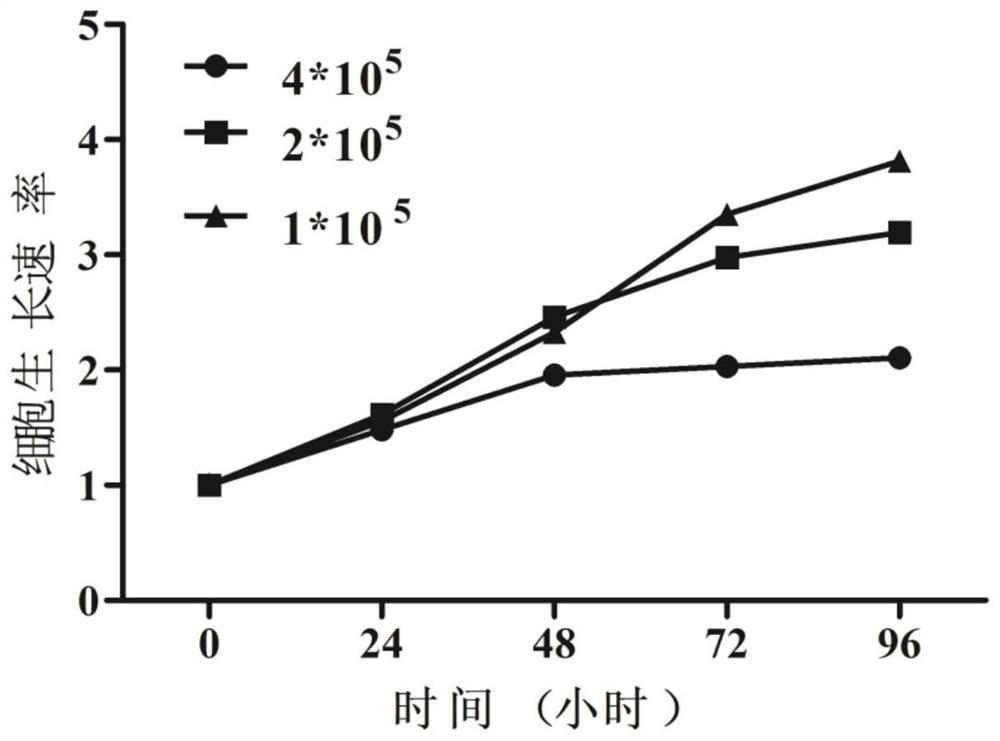
[0032] The invention adopts the IMDM medium containing 10% fetal bovine serum to cultivate the cell line, so that the cell line can grow stably in vitro and be passed down stably. Observed under the microscope, the cells are suspended or weakly attached to the wall, single or clustered, round or oval. Wright-Giemsa staining showed that the cells were mainly primitive erythrocytes, the cells were large, the cytoplasm was dark blue, some had a large number of vacuoles, there were light stained areas around the nucleus, the nuclear chromatin was fine and granular, and there were obvious nucleoli. The cell line did not express CD34 and CD11b by flow cytometry, but highly expressed CD71. Whole-exome sequencing of the cell line cells revealed that the cell line cells were negative for JAK2, CALR, and MPL mutations, and positive for TP53, ASXL1, and IKZF1 mutations in genes related ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com