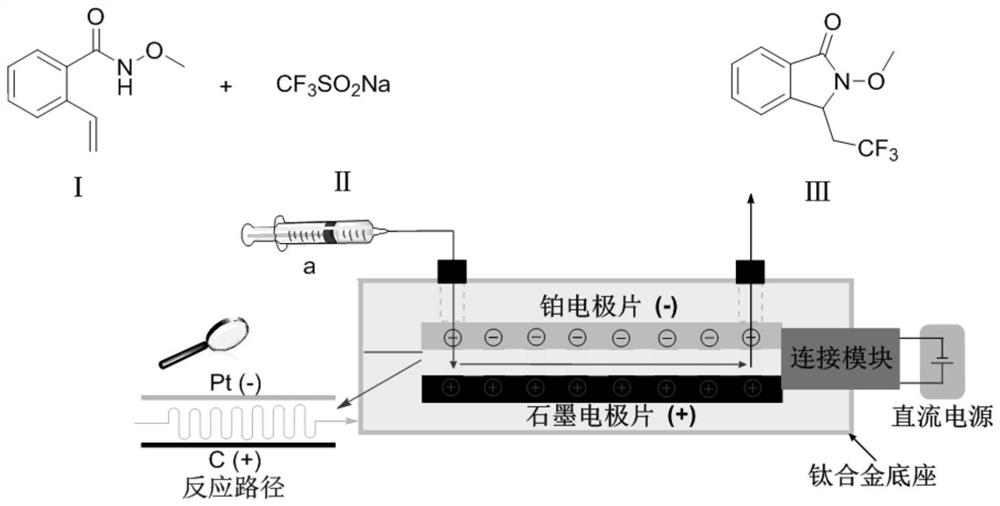
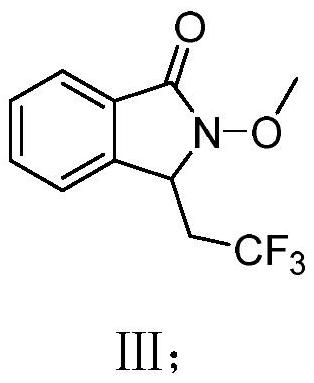
Method for continuous electrosynthesis of isoindolinone by using micro-reaction device
An isoindolinone, electrosynthesis technology, applied in chemical instruments and methods, chemical/physical/physical-chemical reactors, electrolysis processes, etc., can solve problems such as the need for oxidants and harsh reaction conditions, and achieve easy operation and reaction. Mild conditions, the effect of avoiding decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthesis of embodiment 1 compound III:
[0032] Dissolve 0.5mmol (0.0886g) of compound I, 1.5mmol (0.234g) of sodium trifluoromethanesulfinate II and 1mmol (0.302g) of tetrabutylammonium acetate in acetonitrile / water (10mL, volume ratio 4 / 1 , the same below), to obtain a homogeneous solution A, which was added to the syringe pump a; the injection flow rate of the syringe pump a was 225 μL / min; the applied current was 10 mA; the microchannel reactor reaction volume V=225 μL, and the reaction time was 1 min; After one period of reaction in the microchannel reactor, the reaction liquid was collected, and the product yield calculated by HPLC was 84%, and the product III was obtained after separation by column chromatography. 1 H NMR (400MHz, Chloroform-d) δ7.74 (d, J = 7.5Hz, 1H), 7.53 (d, J = 7.6Hz, 1H), 7.44–7.37 (m, 2H), 4.86 (dd, J = 6.7,4.0Hz,1H),3.88(s,3H),2.97–2.78(m,1H),2.55–2.40(m,1H). 13 C NMR (101MHz, Chloroform-d) δ164.7, 140.7, 132.6, 129.3, 129.2, 125.2...
Embodiment 2
[0033] The synthesis of embodiment 2 compound III:
[0034] Dissolve 0.5mmol (0.0886g) of compound I, 1.5mmol (0.234g) of sodium trifluoromethanesulfinate II and 1mmol (0.387g) of tetrabutylammonium hexafluorophosphate in acetonitrile / water (10mL, with a volume ratio of 4 / 1, the same below), to obtain a homogeneous solution A, add in the syringe pump a; the injection flow rate of the syringe pump a is 225 μL / min; the applied current is 10mA; the microchannel reactor reaction volume V=225 μL, and the reaction time is 1min ; After a period of reaction in the microchannel reactor, the reaction liquid was collected, and the product yield was calculated by HPLC to be 77%, and the product III was obtained after separation by column chromatography.
Embodiment 3
[0035] The synthesis of embodiment 3 compound III:
[0036] Dissolve 0.5mmol (0.0886g) of compound I, 1.5mmol (0.234g) of sodium trifluoromethanesulfinate II and 1mmol (0.329g) of tetrabutylammonium tetrafluoroborate in acetonitrile / water (10mL, with a volume ratio of 4 / 1, the same below), to obtain a homogeneous solution A, add in the syringe pump a; the injection flow rate of the syringe pump a is 225 μL / min; the applied current is 10mA; the microchannel reactor reaction volume V=225 μL, and the reaction time is 1min ; After a period of reaction in the microchannel reactor, the reaction liquid was collected, and the product yield was calculated by HPLC to be 74%, and the product III was obtained after separation by column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com