Application of acetylcholin esterase inhibitor in preparation of medicine for treating Alzheimer's disease and preparation method of acetylcholin esterase inhibitor
A technology of inhibitors and drugs, applied in the direction of pharmaceutical formulations, drug combinations, nervous system diseases, etc., can solve problems such as limited options and achieve good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
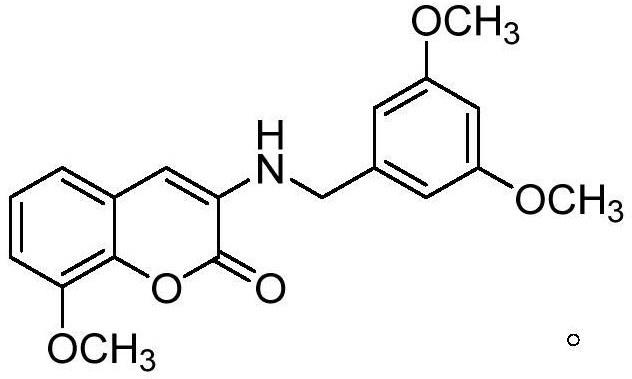
[0030] Example 1 Preparation of 3-((3,5-dimethoxybenzyl)amino)-8-methoxycoumarin (referred to as compound I in the following examples)
[0031]
[0032] Reagents and conditions: (a) methanol, 50°C; (b) concHCl, methanol; (c) potassium carbonate, N,N-dimethylformamide, 102°C.
[0033] Synthetic method of intermediate 3-aminocoumarin: Synthesis of intermediate 3-formamido-8- Methoxycoumarin. Under the condition of magnetic stirring, first add 2-hydroxy-3-methoxybenzoic acid to the methanol solution, then add ethyl isocyanoacetate and pyridine, and finally add cuprous iodide, and put the reaction mixture in a 50°C oil bath Stir and heat in the pot for 6 hours, and monitor the progress of the reaction by TLC. After the reaction, the crude solid compound was obtained by filtration, washed three times with methanol solution, 15 ml each time, and the yield of light yellow solid 3-formamidocoumarin was 85%. Reflux 8-methoxy-3-formamidocoumarin with concentrated hydrochloric acid...
Embodiment 2
[0035] Embodiment 2 pharmacological activity experiment
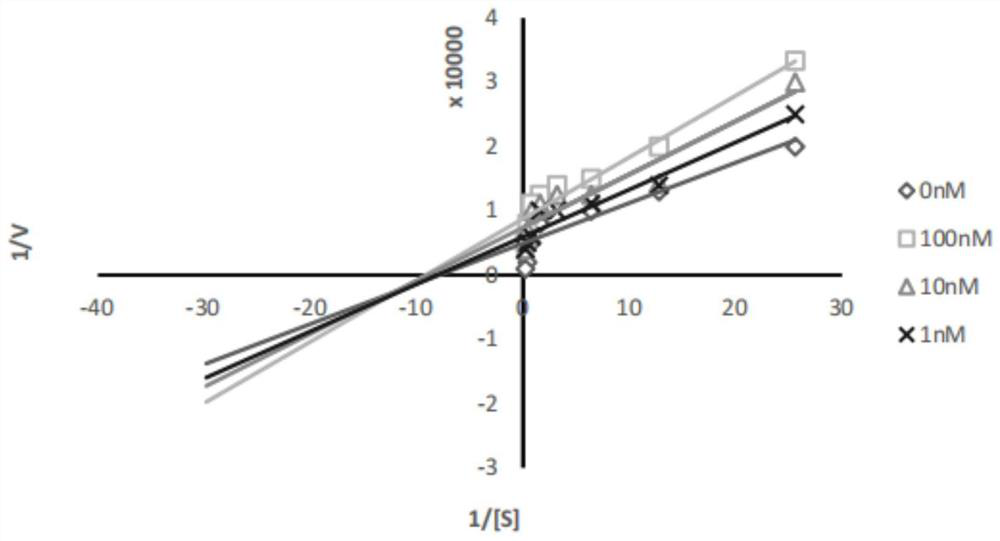
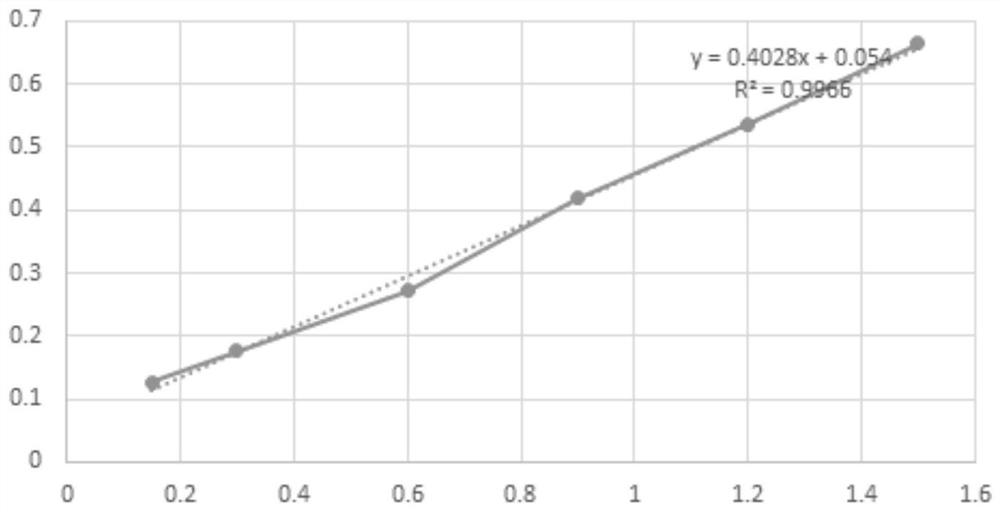
[0036] Determination of 3-((3,5-dimethoxybenzyl)amino)-8-methoxycoumarin in vitro acetylcholinesterase inhibitory activity:
[0037] 1. Preparation of reaction solution
[0038](1) Preparation of PBS buffer solution: Accurately weigh 71.63g of disodium hydrogen phosphate, add an appropriate amount of distilled water to dissolve, then use a volumetric flask to accurately adjust the volume to 1L, and prepare a 0.2mol / L disodium hydrogen phosphate solution; accurately weigh 31.20 g sodium dihydrogen phosphate, add appropriate amount of distilled water to dissolve, then use a volumetric flask to accurately adjust the volume to 1L, and prepare a 0.2mol / L sodium dihydrogen phosphate solution. Accurately measure 473.50mL disodium hydrogen phosphate solution and 26.50mL sodium dihydrogen phosphate solution, mix them, add distilled water and use a volumetric flask to accurately adjust the volume to 1L, and prepare 0.1mol / L, PH=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com