Imidazolonylquinoline compounds and therapeutic uses thereof
A compound and composition technology, applied in the field of imidazolone quinoline compounds and their therapeutic uses, can solve the problems of high lipophilicity, KU-55933 is not suitable for in vivo use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0209] According to another aspect, the present invention provides deuterated derivatives of compound Y. According to one embodiment, the present invention provides:
[0210] ● 8-(1,3-Dimethylpyrazol-4-yl)-1-[3-fluoro-5-(trideuteromethoxy)-4-pyridyl]-7-methoxy-3 -(trideutero-methyl)imidazo[4,5-c]quinolin-2-one (compound 3) and
[0211] ● 1-[3-fluoro-5-(trideuteromethoxy)-4-pyridyl]-7-methoxy-3-methyl-8-[3-methyl-(trideutero-methoxy) yl)pyrazol-4-yl]imidazo[4,5-c]quinolin-2-one (compound 4).
[0212] ● 8-(1,3-Dimethylpyrazol-4-yl)-1-[3-fluoro-5-(trideuteromethoxy)-4-pyridyl]-7-methoxy-3 -Methyl-imidazo[4,5-c]quinolin-2-one (compound 5),
[0213] and its salt.
[0214] Compounds 3, 4 and 5 are represented by the formula:
[0215]
[0216] In other embodiments, the invention provides atropisomers 3-a, 3-b, 4-a, 4-b, 5-a, and 5-b:
[0217]
[0218]
[0219]
[0220] or its salt.
[0221] Consistent with what has been set forth above for compounds 1 and 2, the p...
Embodiment 1
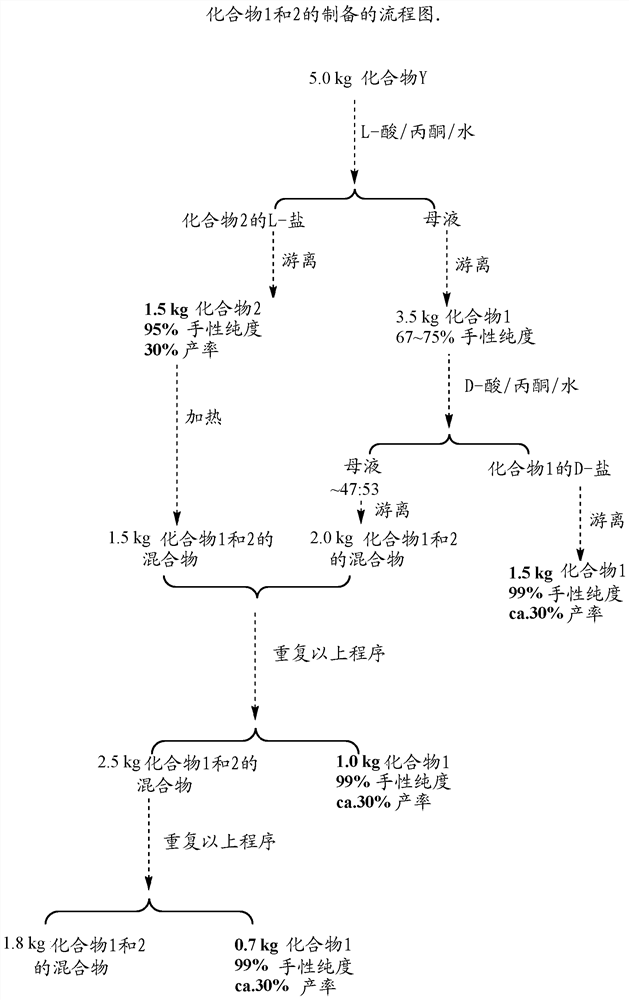
[0433] Example 1: Preparation of Compounds 1 and 2
[0434] Compound Y was prepared according to the procedure disclosed in WO 2016 / 155844, followed by isolation of compounds 1 and 2 from compound Y:
[0435] .
[0436] a. 6-Bromo-N-(3-fluoro-5-methoxy-4-pyridyl)-7-methoxy-3-nitro-quinolin-4-amine Synthesis
[0437] Under dry nitrogen atmosphere, provide 3-fluoro-5-methoxypyridin-4-amine (447 mg, 3.02 mmol) dissolved in N, N - solution in dimethylformamide (5 mL). Then, sodium hydride (504 mg, 12.6 mmol, 60%) was added to the solution and stirring was continued at room temperature for 5 minutes. followed by 6-Bromo-4-chloro-7-methoxy-3-nitro-quinoline (800 mg, 2.52 mmol) was added to the reaction mixture, followed by stirring at room temperature for 15 minutes, then the reaction was quenched by adding ice water (100 mL). The precipitate was filtered off, washed with ice water and dried to give 1.00 g (94%) of 6-Bromo-N-(3-fluoro-5-methoxy-4-pyridine Pyridyl)...
Embodiment 2
[0450] Example 2: Separation of atropisomers and purification of compound 1
[0451] As in Scenario 1 and Image 6 Compounds 1 and 2 can be isolated from compound Y as shown in and discussed in detail below. Those of ordinary skill in the art will understand that the following methods are equally applicable to the isolation of compounds 3-a and 3-b from 3, 4-a and 4-b from 4, and 5-a and 5-b from 5.
[0452] Scheme 1: Preparation of Chiral Salts
[0453]
[0454] step 1:
[0455] 1. In a 200 L reactor, add acetone (108 L, 20 vol), purified water (8.13 L, 1.5 vol) and compound Y (5.42 Kg, 1.0 eq) at 20~25 °C.
[0456] 2. Charge dibenzoyl-L-tartaric acid (4.33 Kg, 1.0 equiv).
[0457] 3. Heat to 52-55°C to get a clear solution, stir at 52-55°C for 0.5 h.
[0458] 4. Cool to 20~25℃.
[0459] 5. Filter and wash the filter cake once with acetone (5.4 L, 1 vol).
[0460] 6. The filter cake was collected and dried to give L-salt B (L-salt of compound 2) (pale yellow s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com