Immunodeficient rodent
A technology for mice and rodents, applied in blood/immune system cells, biochemical equipment and methods, animal husbandry, etc., can solve the problems of rodents and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] The present invention includes a method for preparing a knock-in rodent obtained by knocking in the human G-CSF gene, the method comprising the step of knocking the human G-CSF gene into the G-CSF receptor locus of the rodent.
[0087] Also included is a method for producing a rodent that is the G-CSF receptor locus of the rodent by transplanting human HSCs into a knock-in rodent obtained by knocking in the human G-CSF gene Knock-in rodents in which the human G-CSF gene was knocked in were transplanted with human HSCs that differentiated and circulated human neutrophils in the periphery.
[0088] The present invention also includes a knock-in rodent obtained by knocking in the human G-CSF gene in the G-CSF receptor locus of the rodent, transplanted with human HSC, and peripherally differentiating and circulating human neutrophils .
[0089] In the present invention, rodents are not limited, and examples thereof include mice, rats, guinea pigs, hamsters, rabbits, nutria...
Embodiment 1
[0144] Example 1: Human G-CSF knock-in mice differentiated from human neutrophils
[0145] 1. Preparation of KI targeting carrier
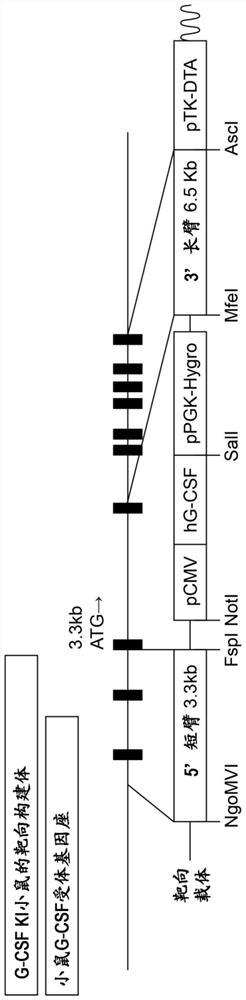
[0146] The human G-CSF gene is arranged downstream of the cytomegalovirus (CMV) promoter as a systemic expression promoter adjacently, and the homologous sequence 3.3Kb upstream and 6.5Kb downstream of the mouse G-CSF receptor region is clamped Hold, prepare KI targeting carrier. The structure of the targeting vector is shown in figure 1 middle.
[0147] 2. Preparation of human G-CSF knock-in mice
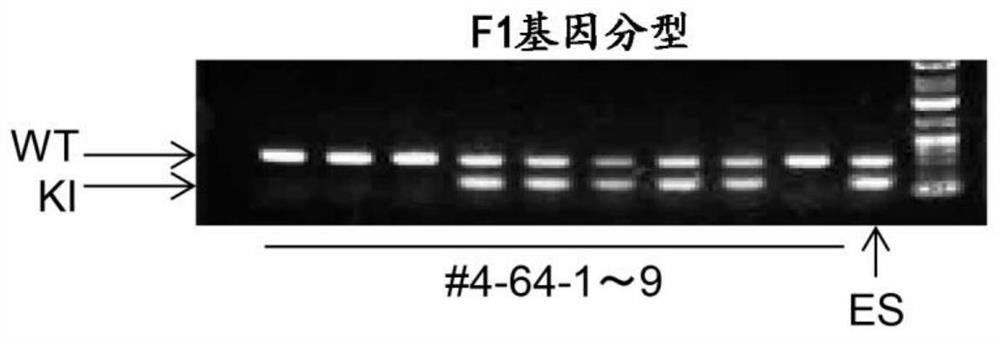
[0148] Electroporate NOG mouse ES cells with the targeting vector prepared above to establish homologous recombination ES cells, then prepare chimeric mice, and then establish F1 mice by mating with NOG mice, and use PCR Analysis of G-CSF receptor gene. figure 2 The results of genetic analysis are shown in . The established mouse system is formally known as NOD.Cg-Prkdc scid Il2rg tm1Sug wxya tm1(CMV-CSF3) / Jic, the official strain name, abbr...
Embodiment 2
[0183] Example 2: Hyperactivity of human neutrophils by mating with FcR KO mice
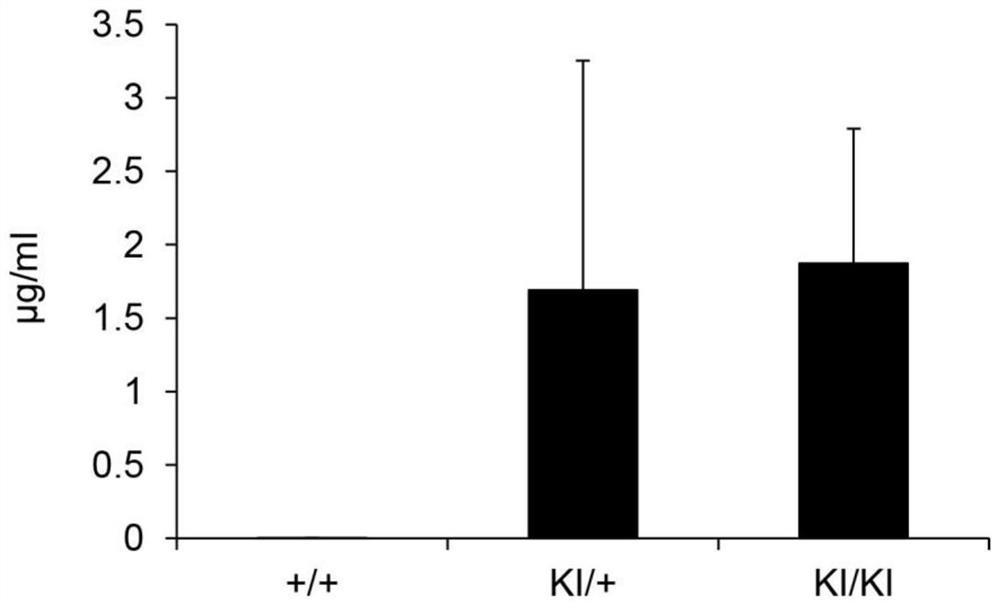
[0184] FcRKO mice lacking Fcer1g and Fcgr2b, which are constituent molecules of receptors that bind to the Fc region of antibodies, have been established (Inoue Y et al., Journal of Immunology 2007; 179:764-774.). The newly established hG-CSF KI, FcR KO mice obtained by mating this FcR KO mice with hG-CSF KI mice were transplanted with human hematopoietic stem cells, and the results showed that the human leukocytes in the peripheral blood of the mice The proportion of human neutrophils in hG-CSF KI mice was about 2 to 5%, whereas in hG-CSF KI, FcR KO mice, it increased about 4 times to 15 to 20% ( Figure 19 ). Neutrophils differentiate from hematopoietic stem cells in a G-CSF-dependent manner in the bone marrow, and in response to stimuli such as infection, hyperdifferentiate, and at the same time rapidly migrate to the site where the stimuli entered, thereby being able to eliminate bacteria. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com