Novel torasemide impurity and preparation method thereof
A technology of torasemide and impurities, which is applied in the field of new impurities of torasemide and its preparation, and can solve problems such as the difficulty of complete removal of impurities, the influence of drug safety and effectiveness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of TL-Imp07 intermediate
[0030] Condensation reaction of 4-chloro-3-pyridine sulfonamide with 4-chloro-3-pyridine sulfonyl chloride and ammoniation reaction with m-toluidine to obtain crude product separated by column chromatography to obtain pure impurity product
[0031] Add 4-chloro-3-pyridinesulfonamide (20g), 4-chloro-3-pyridinesulfonyl chloride hydrochloride (28g) and dichloromethane (200ml) into a 500ml round bottom flask and start stirring, then add potassium carbonate (30g), continue to stir at room temperature for about 4~5h, filter, add 100ml of water to the filtrate, separate the liquid, extract the organic phase with water twice, dry with anhydrous sodium sulfate and concentrate to dryness to obtain the TL-Imp07 intermediate.
Embodiment 2
[0032] Embodiment 2: Preparation of TL-Imp07
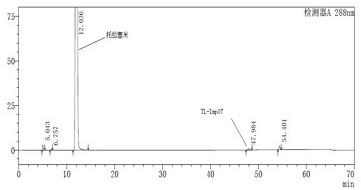
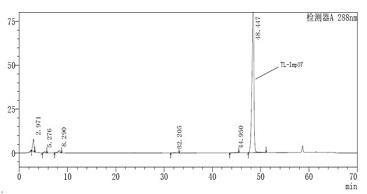
[0033] Add the TL-Imp07 intermediate obtained above, m-toluidine (30g) and N,N-dimethylformamide (200ml) into a 500ml round bottom flask, start stirring, add potassium carbonate (40g) and heat to 80°C Then continue to react for about 24h, cool down to room temperature and concentrate to dryness. The obtained crude product is dissolved in methanol (30ml) and added with silica gel (20g) and then concentrated to dryness. TL-Imp07 (9.65 g, HPLC purity 97.1%) was obtained by analysis.
Embodiment 3
[0034] Embodiment 3: the preparation of TL-Imp07 intermediate
[0035] Condensation reaction of 4-chloro-3-pyridine sulfonamide with 4-chloro-3-pyridine sulfonyl chloride and ammoniation reaction with m-toluidine to obtain crude product separated by column chromatography to obtain pure impurity product
[0036] Add 4-chloro-3-pyridinesulfonamide (20g), 4-chloro-3-pyridinesulfonyl chloride hydrochloride (28g) and dichloromethane (200ml) into a 500ml round bottom flask and start stirring, then add triethyl Amine (20ml), continue to stir at room temperature for about 4~5h, filter, add 100ml of water to the filtrate, separate the liquid, extract the organic phase with water twice, dry with anhydrous sodium sulfate and concentrate to dryness to obtain the TL-Imp07 intermediate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com