Protopanoxadiol derivative as well as preparation method and application thereof
A technology of protopanaxadiol and derivatives, applied in the direction of drug combination, pharmaceutical formula, steroid compound, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of the protopanaxadiol valine ester of embodiment 1.Boc protection
[0025]
[0026] Dissolve 1.89g of Boc-protected valine in 40mL of Py, then add 1.66g of EDCI into it, activate it at room temperature for half an hour, then add 0.80g of DMAP, 1g of protopanaxadiol in sequence, and raise the temperature after the addition The reaction was carried out at 40°C. After reacting for 10 hours, the reaction solution was concentrated under reduced pressure to remove part of pyridine, and the remaining viscous material was added to 50 mL of EA, then washed twice with 50 mL of 10% dilute hydrochloric acid, and washed twice with 50 mL of saturated Na 2 CO 3 The solution was washed twice, and washed once with 40 mL of saturated brine, and the organic layer was dried over anhydrous sodium sulfate. Suction filtration and concentration under reduced pressure gave 1.8 g of a yellow solid.
Embodiment 2
[0027] Embodiment 2. The preparation of protopanaxadiol valine ester
[0028]
[0029] Dissolve 0.8g of the solid obtained in the previous step in a mixed solvent of 6mL toluene and 4.5mL isopropanol, replace the argon protection, drop the temperature to -10°C, add 0.2mL methanesulfonic acid dropwise, and react at 50°C for eight hours , adding 10 mL of EA, followed by washing with 10 mL of saturated sodium carbonate solution three times, and once with 10 mL of saturated brine, and drying the organic layer over anhydrous sodium sulfate. Suction filtration and concentration under reduced pressure gave 0.6 g of the crude product, which was eluted with ethyl acetate / petroleum ether (16%–50%) through silica gel column chromatography to obtain 0.3 g of 3-Val-PPD protopanaxadiol valine derivative. g, yield 44%, and C 20 0.35g of dehydrated 3-Val-PPD protopanaxadiol valine derivative. The yield is 50%.
Embodiment 3
[0030] Embodiment 3. The preparation of protopanaxadiol valine ester hydrochloride
[0031]
[0032] Purified 0.3g 3-Val-PPD and C 20 The dehydrated 0.35g 3-Val-PPD protopanaxadiol valine derivative was dissolved in ethyl acetate, added the ethyl acetate solution of HCl, precipitated, and was filtered by suction to obtain the corresponding protopanaxadiol valine Acid derivative hydrochloride 0.3g and C 20 0.35 g of dehydrated protopanaxadiol valine derivatives.
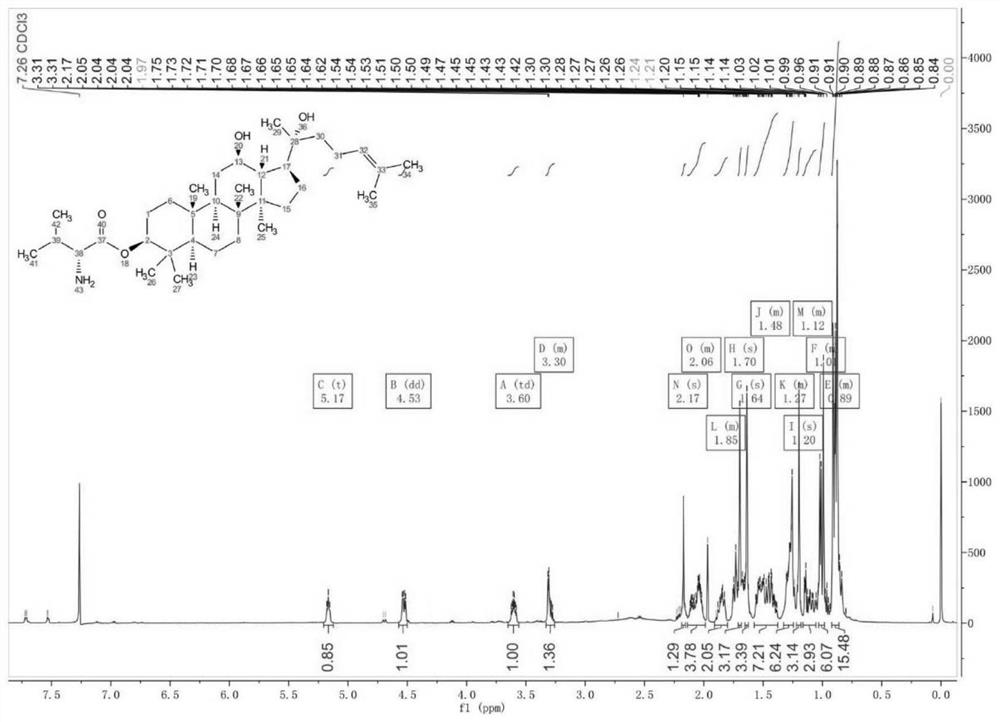
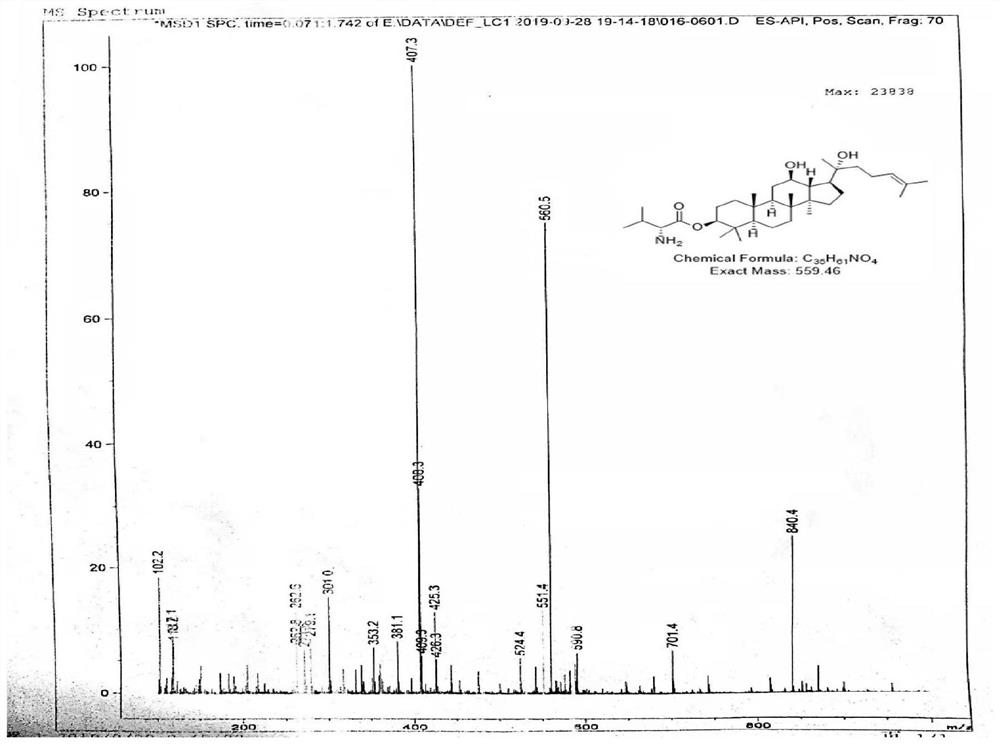
[0033] 3-Val-PPD proton spectrum and mass spectrum data are as follows:
[0034] 1 H-NMR (600MHz, CDCl 3 ): δ5.17(t, J=7.2Hz, 1H), 4.53(dd, J 1 =11.6Hz,J 2 =4.9Hz,1H),3.60(td,J 1 =10.4Hz,J 2 =5.2Hz,1H),3.33-3.26(m,1H),2.17(s,1H),2.14-1.99(m,4H),1.91-1.80(m,2H),1.70(s,3H),1.64( s,3H),1.58-1.38(m,7H),1.33-1.24(m,6H),1.20(s,3H),1.16-1.06(m,3H),1.03-0.98(m,6H),0.92- 0.86(m,15H).ESI-MS(m / z):560.5[M+H] + .
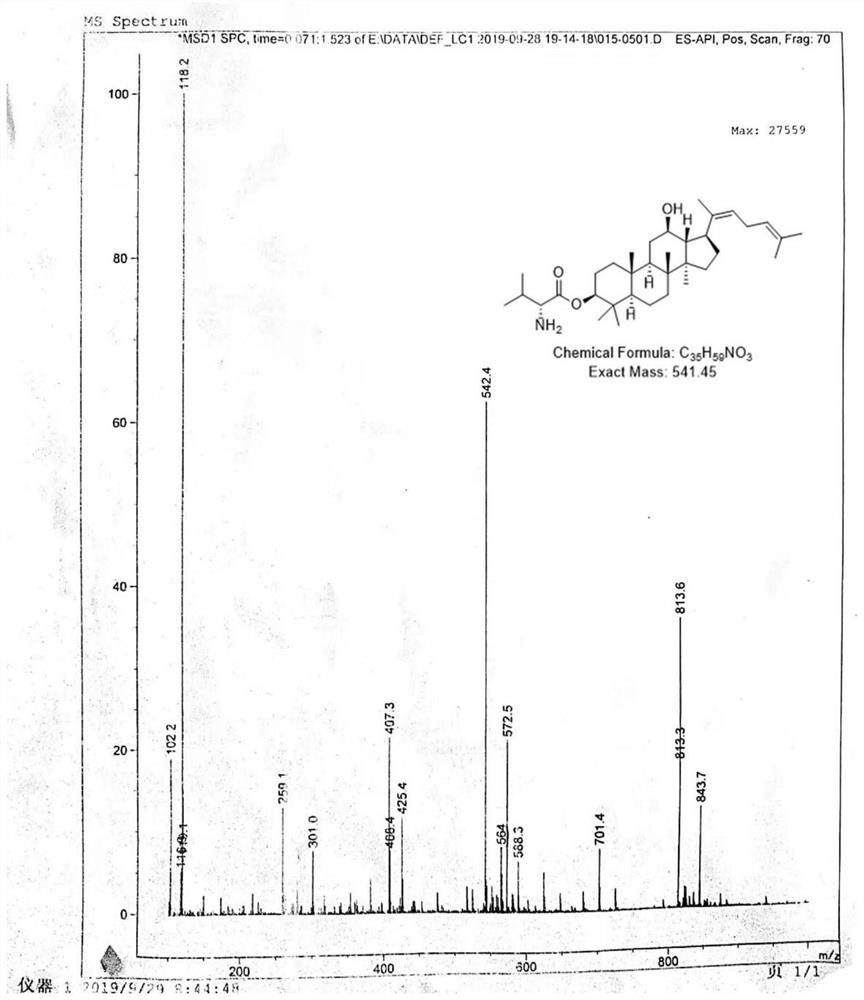
[0035] C 20 -dehydrated 3-Val-PPD: ESI-MS(m / z): 542.4[M+H] + .
[0036] 3,12-diVal-PPD: ESI-MS(m / z)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com