Monoterpenes for treating respiratory tract diseases, in particular bronchopulmonary diseases
a technology of respiratory tract diseases and monoterpenes, applied in the field of medicine, can solve the problems of insufficient deposition of inhalative respiratory therapeutics in the peripheral respiratory tract, unwanted side effects, and inability to achieve the effect of reducing the risk of pulmonary artery disease,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working examples
Example 1
In-Vitro Studies
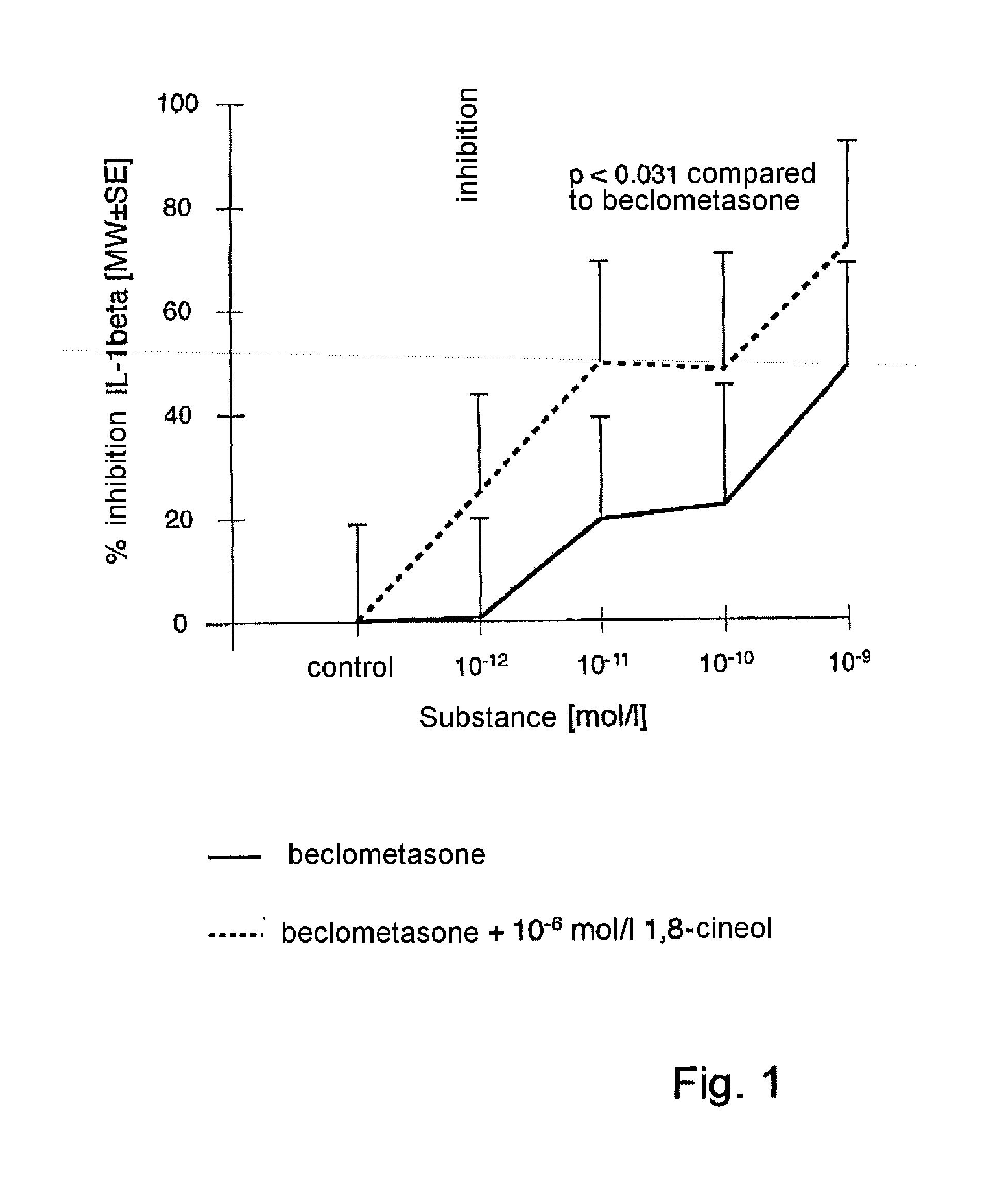
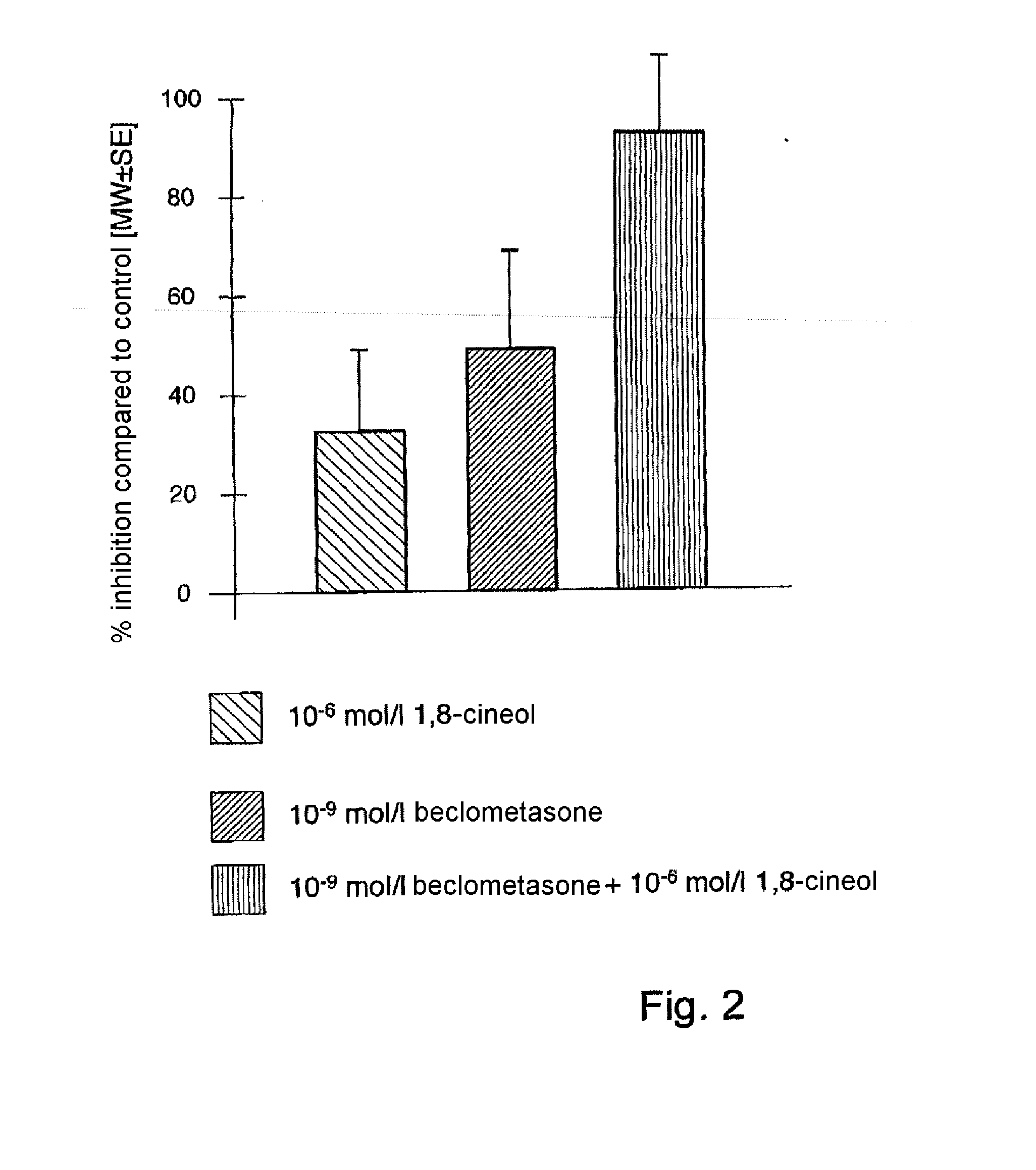
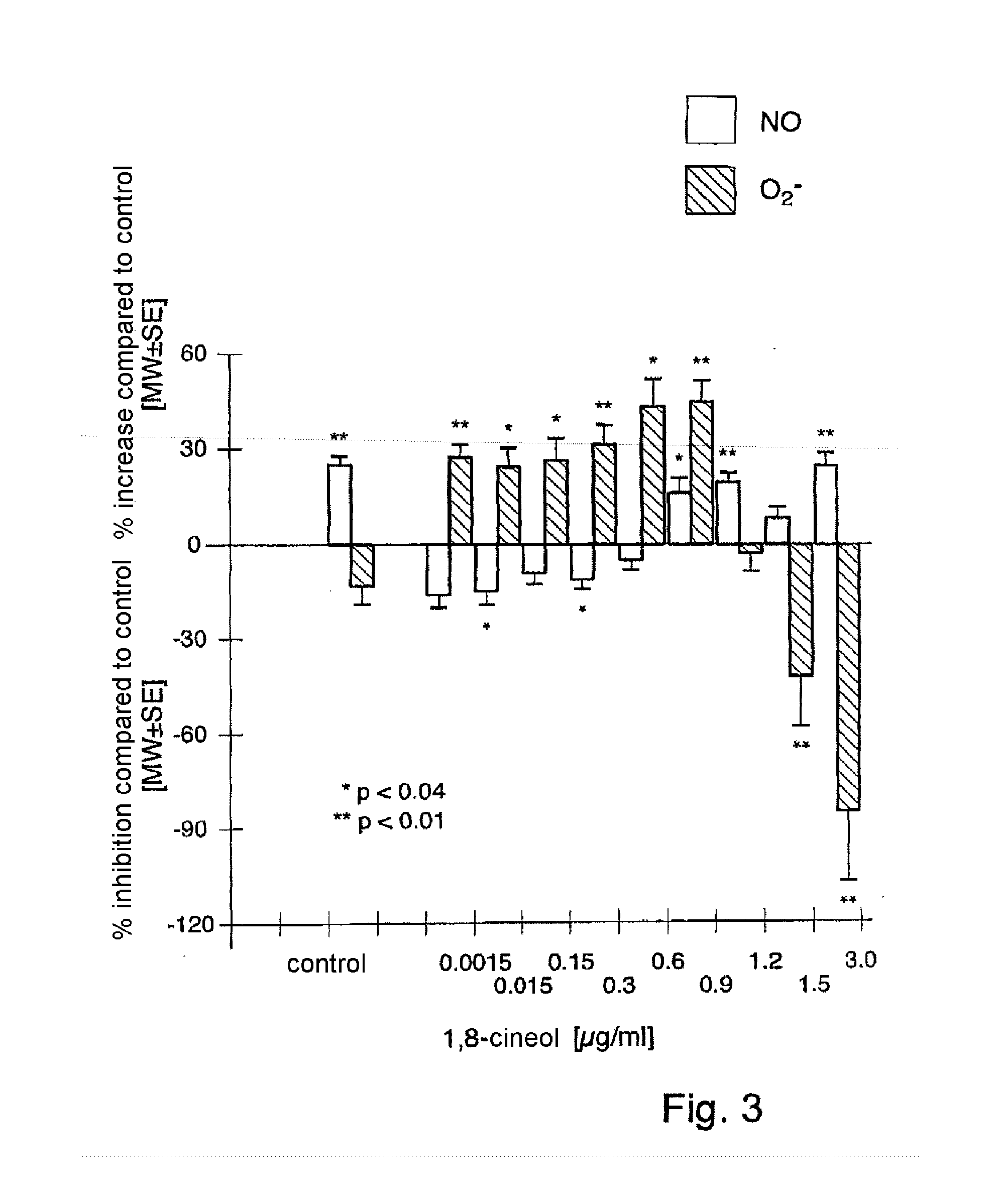
[0111]In in vitro studies, it was found that monoterpenes (here specifically: 1,8-cineol) are capable of enhancing the activity of inhalative respiratory therapeutics, in particular glucocorticoids (here specifically: beclometasone), in a significant, in particular synergistic, manner.
[0112]Surprisingly, it has been found that therapeutically relevant concentrations of 1,8-cineol significantly enhance the anti-inflammatory action of topical glucocorticoids by synergistic inhibition of cytokine production (see Table 1).
[0113]Table 1 shows the synergistic activity of 1,8-cineol (10−6 mol / l) and beclometasone on the LPS-stimulated production of IL-1beta in human monocytes in vitro. Monocyte IL-1beta production (n=14-15, 4 experiments) in monocytes is inhibited significantly by cineol (10−6 mol / l) compared to the control. Cineol and beclometasone synergistically inhibit IL-1beta production more than beclometasone on its own. Compared to cineol (10−6 mol / l) on it...
example 2
Clinical Results
[0128]10 patients 56 to 72 years of age with persistent bronchial asthma (GINA II) treated with a combination therapy of inhalative glucocorticoid (beclometasone, 2×200 μg / die by inhalation) and inhalative long-acting beta-2-sympathomimetics (LABA, salmeterol) and also oral theophyllin were given 1,8-cineol (Soledum® capsules) 4×200 mg / die orally for one week. Even after a one-week therapy at the dose mentioned above, in 8 of the 10 subjects a slight to moderate improvement of lung function was achieved. After continuing therapy for a further twelve weeks, the persistent bronchial asthma had stabilized to such an extent that in 7 of the 10 patients the inhalative glucocorticoid required could be reduced by up to 60% and in 2 of the 10 patients the inhalative glucocorticoids could be discontinued altogether at times. The therapy was tolerated well, without any side-effects. In 5 of the patients treated, the betamimetics required, too, could be reduced by up to 40%.
[01...
example 3
Further Clinical Results
[0131]In a placebo-controlled double-blind study, the effect of an additional therapy with 1,8-cineol in the form of capsules which dissolve in the small intestine (Soledum® capsules, 3×200 mg / die oral) on exacerbation rate and lung function was examined in 3 winter months of two successive years using 242 smokers with COPD (GOLD II to III). Both patient groups were identical with respect to age, sex, body mass index, smoker status, lung function and a guideline-conform medication of ICS, LABA, SABA, anticholinergics and theophyllin. In the verum group, the additional, therapy with 1,8-cineol led to a significant reduction in the exacerbation rate of −38.5% compared to the placebo group. Additionally, the lung function (FEV1) in the verum group (+5.1%) had improved significantly compared to the placebo group (−1%). Clinical parameters such as the St. George's Respiratory Questionnaire (SGRQ), too, had improved significantly more in the verum group (−10.4 unit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| disorder | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com