Survivin dual inhibitor loaded ferritin nanoparticles as well as preparation method and application thereof
A nanoparticle and inhibitor technology, applied in the field of medicine, can solve the problems of inability to effectively inhibit Survivin protein, systemic toxicity, etc., and achieve the effect of being suitable for large-scale continuous production and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
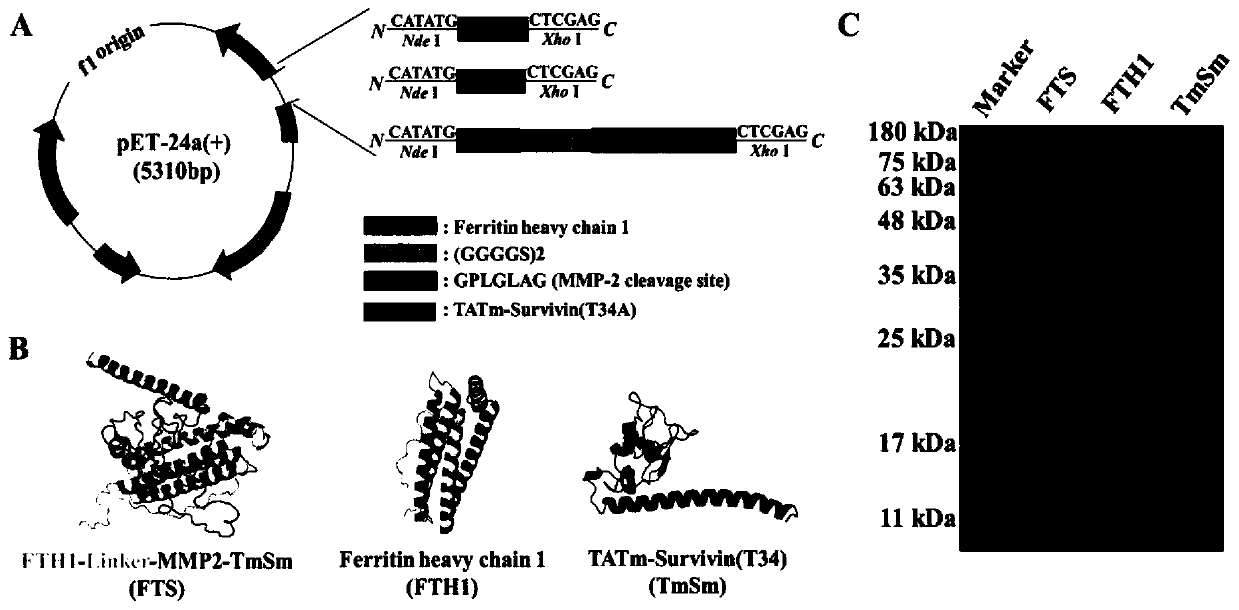
[0041] Example 1. Construction of recombinant plasmids and expression and purification of recombinant proteins
[0042] Reagents and kits: Premix Taq DNA polymerase, Pyrobest DNA polymerase, restriction enzymes (Ned I, Xho I), protein molecular weight standard Premixed Protein Marker (Low), protein loading buffer 4×Protein SDS PAGE Loading Buffer, DNA marker was purchased from Treasure Bioengineering Co., Ltd. (Dalian). Isopropyl-B-D-thiogalactopyranoside (IPTG) and kanamycin were purchased from Sigma-Aldrich (USA), and SPSepharose FF cation exchange columns were purchased from GE (USA). Other biochemical reagents belong to domestic conventional analytical reagents.
[0043] Strains and plasmids: E.coli DH5α and BL21(DE3) (Invirogen, USA) were used as plasmid cloning and expression strains respectively, the recombinant plasmid pET-24a-TmSm was constructed in our laboratory, and pGEM-FTH1 was purchased from Beijing Yiqiao Shenzhou Biotechnology Co., Ltd. Ltd., pET-24a(+) was ...
Embodiment 2
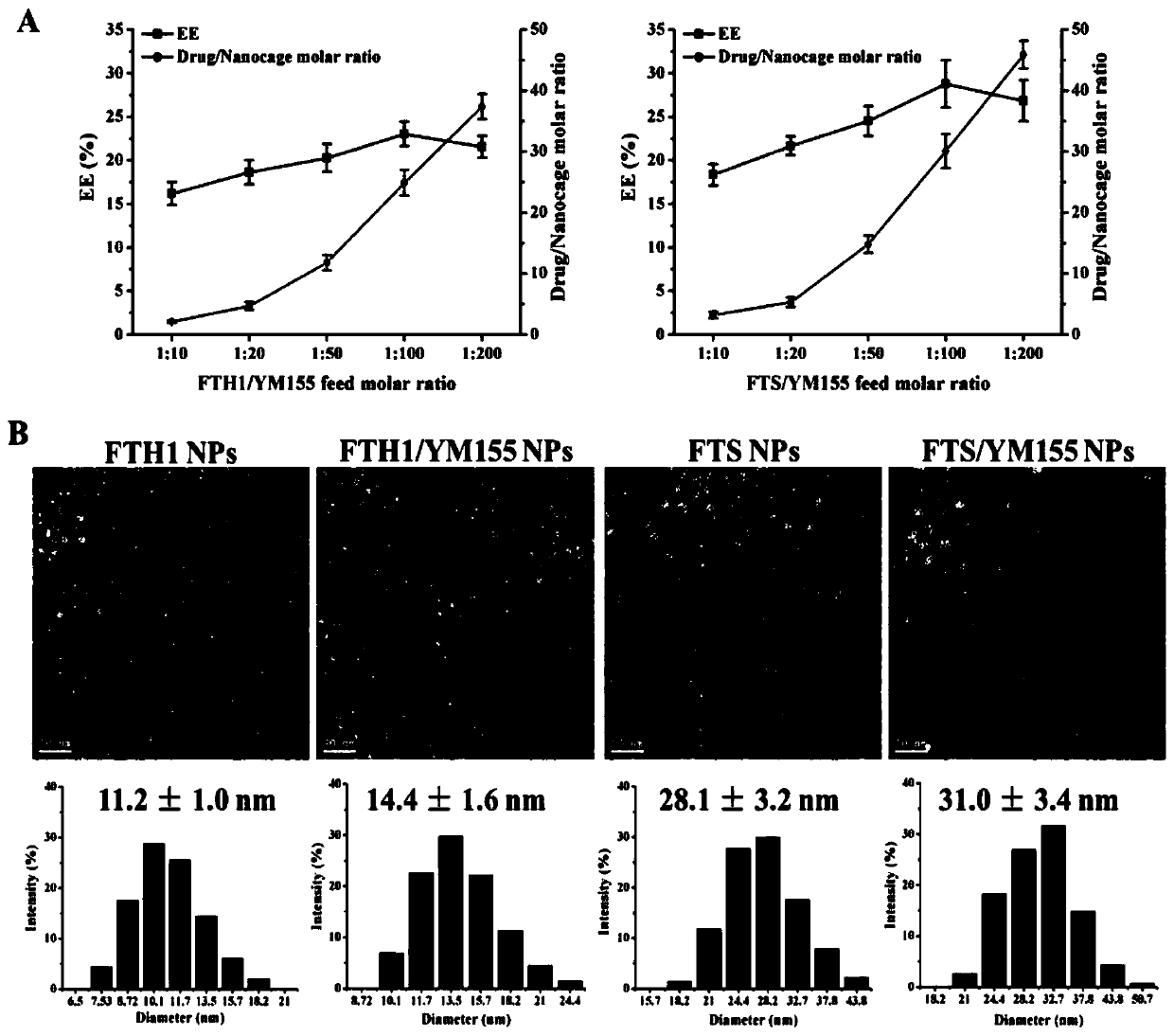
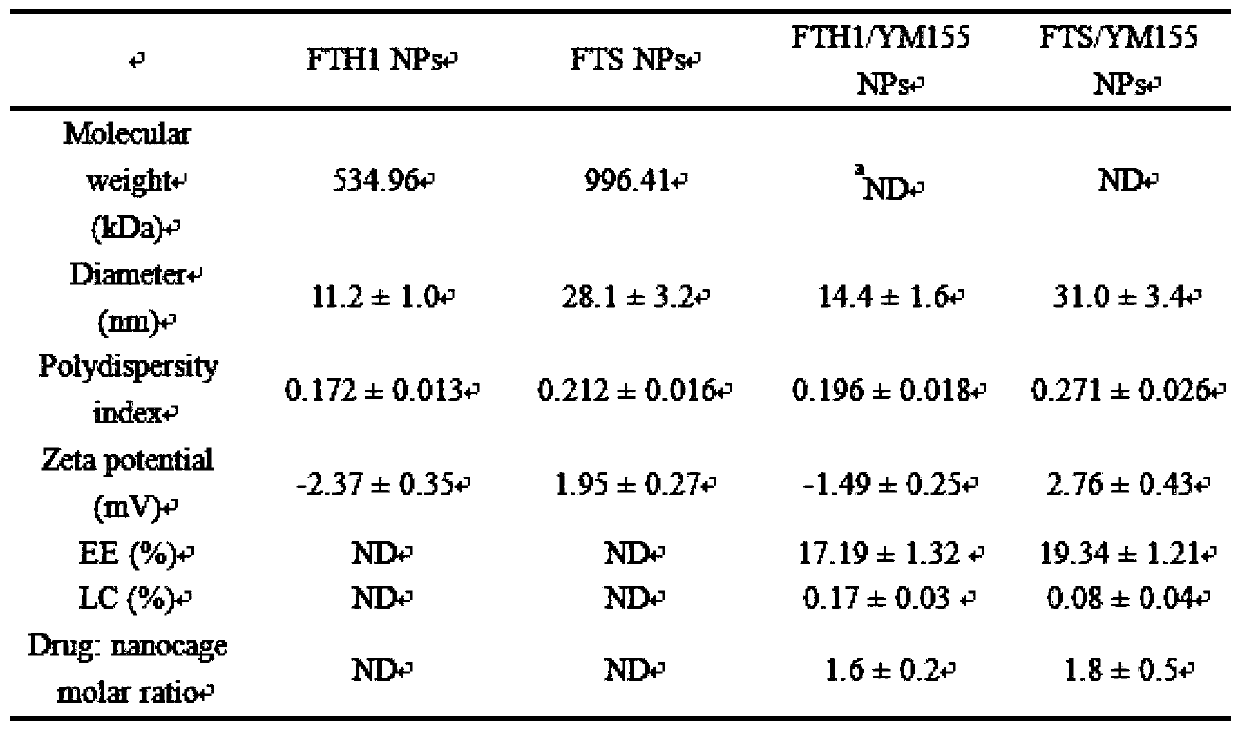
[0092] Example 2. Preparation and characterization of nanoparticles loaded with YM155
[0093] Reagents: YM155 and doxorubicin (DOX) were purchased from Aladdin Biochemical Technology Co., Ltd., and the Bradford method protein concentration determination kit was purchased from Sangon Bioengineering Co., Ltd. (Shanghai). Other biochemical reagents belong to domestic conventional analytical reagents.
[0094] Reagent preparation: related solutions were prepared with reference to the reagents in Example 1.
[0095] Preparation of YM155-loaded nanoparticles
[0096] UV-Vis full-wavelength scanning: Before choosing whether to denature ferritin with acid or alkali, the stability of the embedded substance YM155 in acid-base media was analyzed by UV-Vis spectroscopy. Dilute 1 mL of YM155 standard solution (100 μmol / L) with PBS (pH=2 and 12) to 10 mL, and use PBS as a blank control, and perform full-wavelength scanning in the range of 200-800 nm with a UV-visible spectrophotometer. ...
Embodiment 3
[0108] Example 3. Detection of anti-tumor activity in vivo and in vitro of FTS / YM155NPs
[0109] Reagents and kits: MTT, penicillin and streptomycin mixed solution, DMSO were purchased from Beijing Suolaibao Company; RPMI-1640 medium, fetal bovine serum, and trypsin were purchased from Gibco Company (USA); anti-survivin monoclonal antibody ( Rabbit origin), anti-MMP2 monoclonal antibody (rabbit origin), anti-βactin monoclonal antibody (mouse origin), goat anti-rabbit / goat anti-mouse IgG-HRP secondary antibody were purchased from Proteintech Group (USA). Other biochemical reagents belong to domestic conventional analytical reagents.
[0110] Cell lines and animals:
[0111] Human lung adenocarcinoma cell A549 and human pancreatic cancer cell Capan-2, cultured at 37°C, 5% CO 2 Concentration, the medium is RPMI1640 medium (containing 10% fetal bovine serum). Balb / c-nu female nude mice (4-6 weeks old, 20±2g) were purchased from Shanghai Slack Experimental Animal Co., Ltd.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com