Cancer combined treatment composition
A technology of combined therapy and composition, applied in the direction of drug combination, cancer antigen components, breast cancer vaccine, etc., can solve the problems of immunosuppressive microenvironment limitation, no therapeutic effect, no significant improvement of curative effect, etc., to achieve the solution of immune resistance Inhibit and reduce the inhibition of tumor microenvironment and improve the effect of anti-tumor immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] This example is the preparation method of the CAR-T cells of the present invention.
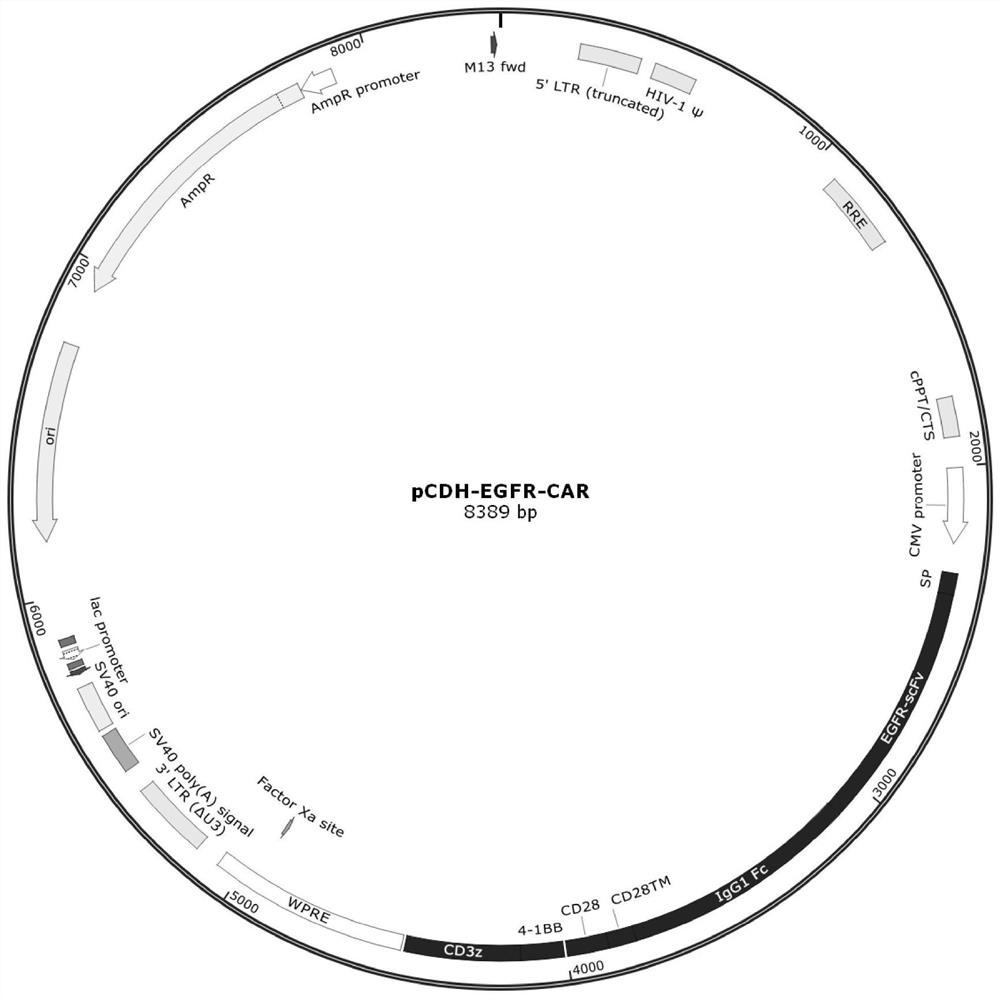
[0064] (1) Packaging and preparation of lentivirus: Two days before transfection, 293T was mixed with 2.5×10 6 Spread on a 10cm plate and culture with 8mL complete medium DMEM+10%FBS. 2-4 hours before transfection, the 293T medium was replaced with 7 mL complete medium RMPI 1640+10% FBS. During transfection, 84 μL PEI was dissolved in 600 μL basal medium RMPI 1640 and allowed to stand for 2 min; then 42 μg plasmid was dissolved in 600 μL basal medium RPMI 1640, in which the EGFR-CAR recombinant transfer vector based on the pCDH vector (such as figure 1 As shown, see CN110845623A), the ratio of psPAX and pMD2G was 4:3:1, then PEI solution was added to the plasmid solution, shaken for 8 seconds immediately, and added to 293T cells after standing for 8 minutes. After 12 hours of transfection, the 293T medium was replaced with 12 mL of complete medium DMEM+10% FBS. After 60 hours of tra...
Embodiment 2
[0068] This example is for the screening of epigenetic regulators in combination therapy for different tumors.
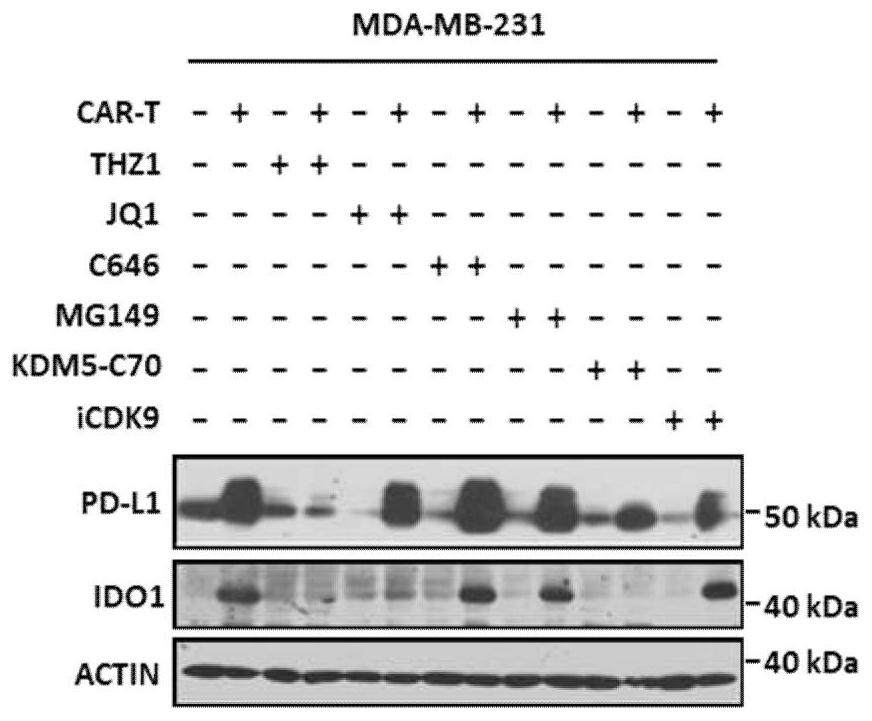
[0069] The tumor cells cultured in vitro (triple-negative breast cancer cell MDA-MB-231 or brain glioma cell U87) were digested and placed in a 6-well plate for overnight culture. When the cell confluence reached 80% the next day, the culture was replaced. At the same time, add the CAR-T cells prepared in Example 1 (effect-to-target ratio E:T=1:2, use X-VIVO medium to suspend), and / or add different epigenetic regulators (250nM THZ1, 100 nM of JQ1, 20 μM of C646, 200 μM of MG149, 5 μM of KDM5-C70, 5 μM of ICDK9, dissolved in DMSO). After 48 hours of co-cultivation, remove the suspended CAR-T cells, use Eastep Super TotalRNA Extraction Kit (Promega) to extract the total RNA of tumor cells, and perform RT-qPCR detection; or after 72 hours of co-cultivation, remove the suspended CAR-T cells , tumor cells were lysed with RIPA lysate, the supernatant was obtained by ultr...
Embodiment 3
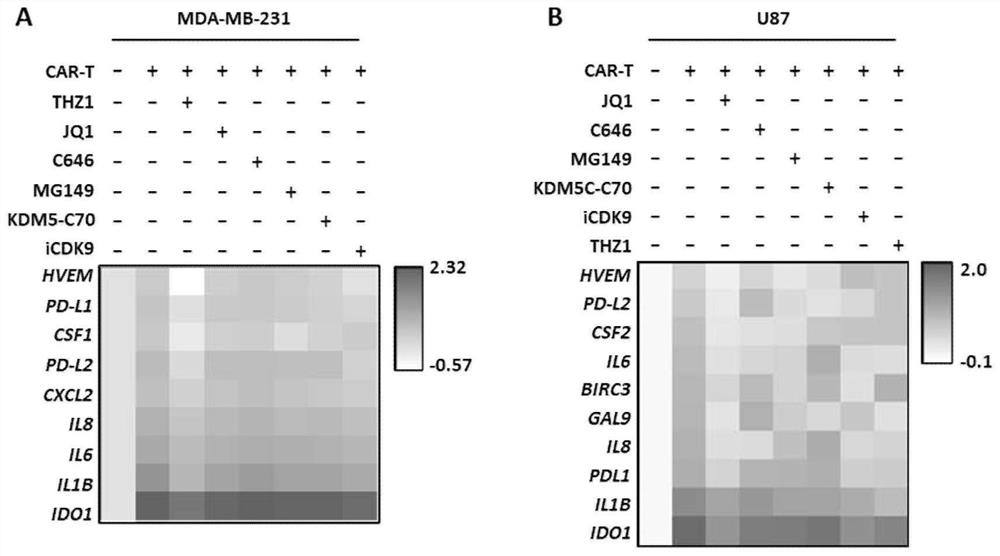
[0073] In this example, the combination of CAR-T and epigenetic regulators targeting CDK7 or BRD4 was tested on the transcriptional effects of various inhibitory immune checkpoints, inflammatory factors, immunosuppressive molecules and chemokines and other molecules related to immune escape. level down. Digest triple-negative breast cancer cells (MDA-MB-231 or MDA-MB468) and glioma cells (U87 or GBM-PDX) cultured in vitro, spread them in 6-well plates and culture overnight, and wait for the cells to reach confluence the next day When the degree reaches 80%, replace the medium and add the CAR-T cells prepared in Example 1 (effect-to-target ratio E:T=1:2, use X-VIVO medium to suspend), and / or add different apparent Genetic regulators: commonly used epigenetic regulators of CDK7 (250nM THZ1, 350nM THZ2, 1μM BS-181, 1μM CT7001) and BRD4 commonly used epigenetic regulators (100nM JQ1, 1μM IBET-151, Molibresib at 500 nM, Mivebresib at 500 nM, INCB057643 at 500 nM, Birabresib at 75 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com