Application of combined medication of bacteroides fragilis and PD-1 or PD-L1 antibody to treatment of genitourinary system cancer
A Bacteroides fragilis, PD-L1 technology, applied in the direction of antibody medical components, antibodies, applications, etc., can solve the problem of no PD-1/PD-L1 antibody microecological preparations, etc., to enhance the body's anti-tumor immune response, The effect of improving immune cell status
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 Bacteroides fragilis living bacteria liquid, the preparation of inactivated bacteria liquid
[0051] Streak inoculation of Bacteroides fragilis ZY-312 strain on blood plate, anaerobic culture for 48h. Observe the colony morphological characteristics, staining characteristics, size, club shape and distribution, etc.
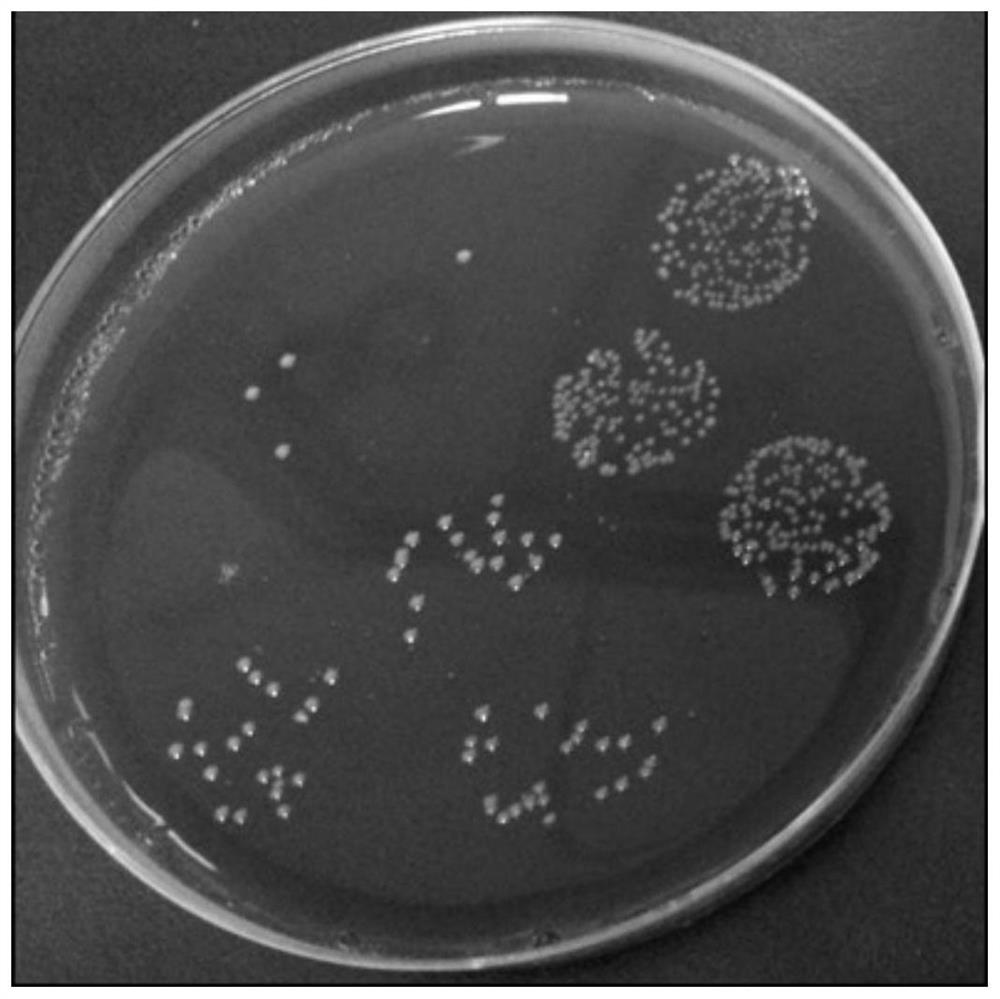
[0052] Colony characteristics: After Bacteroides fragilis ZY-312 was cultured on a blood plate at 37°C for 48 hours, it was slightly convex, translucent, white, smooth, non-hemolytic, and the diameter of the colony was between 1mm and 3mm. See figure 1 .
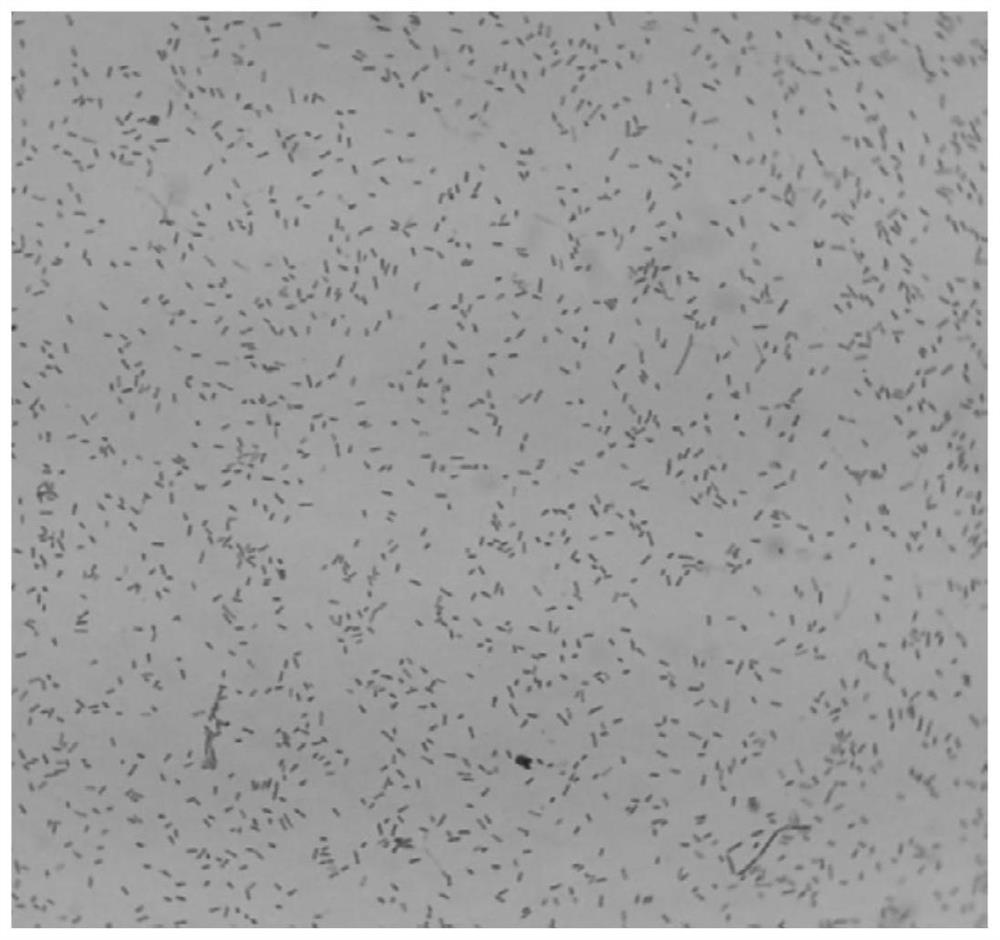
[0053] Morphology under the microscope: Bacteroides fragilis ZY-312 was examined by Gram staining. It is a Gram-negative bacterium with a typical rod shape, blunt rounded ends and dense staining. The uncolored part in the middle of the bacteria is like a vacuole. figure 2 .
[0054] A single colony was selected and inoculated in a plant-derived peptone liquid medium for fermentation and c...
Embodiment 2
[0056] Example 2 Bacteroides fragilis combined with PD-1 antibody in the treatment of 4T1 breast cancer xenografts in mice
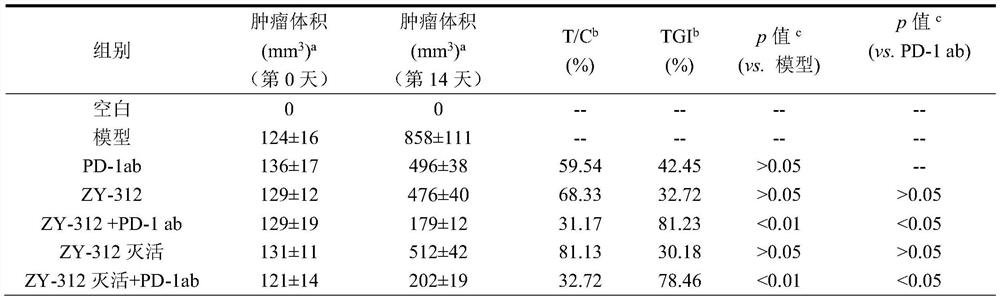
[0057] Experimental design: 70 BALB / c female mice were selected and randomly divided into 7 groups according to body weight range, namely blank group, model group, ZY-312 (10 10 CFU / piece), PD-1 antibody (PD-1ab) group (product number BE0146, purchased from BioXcell, the same 200 μg / piece), ZY-312 live bacteria combined with PD-1 antibody group, ZY-312 inactivated bacteria group (10 10 cell / rat), ZY-312 inactivated bacteria combined with PD-1 antibody group, 10 rats in each group. Except the blank group, animals in other groups were inoculated with 1×10 6 4T1 cells, the tumor volume reaches 100-150mm 3 Dosing in groups was started at D0: starting from D0, animals in the blank group and the model group were orally administered 300 μL of normal saline daily, and intraperitoneally injected with 200 μL of PBS twice a week; The administration volume of th...
Embodiment 3
[0096] Example 3 Bacteroides fragilis combined with PD-1 antibody in the treatment of mouse ID8 ovarian cancer ascites tumor
[0097] 1. Experimental design and process
[0098] 70 C57BL / 6 female mice aged 4-6 weeks were randomly divided into 7 groups according to the weight range, namely blank group, model group, ZY-312 (10 10 CFU / body), PD-1 antibody (PD-1ab) group (BE0273, BioXcell 200μg / piece), ZY-312 live bacteria combined with PD-1 antibody group, ZY-312 inactivated bacteria group (10 10 CFU / only), ZY-312 inactivated bacteria combined with PD-1 antibody group (10 10 CFU / only), 10 in each group.
[0099] Using DMEM culture solution containing 10% calf serum, penicillin (100U / mL) and streptomycin (100U / mL) under normal conditions (37°C, saturated humidity, 5% CO 2 ) to culture ID8 ovarian cancer cells to the logarithmic growth phase, adjust the cell concentration to 2×10 7 cells / mL, except for the blank group, each mouse in each group was intraperitoneally injected wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com