Plasma miRNA markers and application thereof
A technology of markers and plasma, applied in the field of plasma miRNA markers, preparation kits and kits, can solve the problem of large trauma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
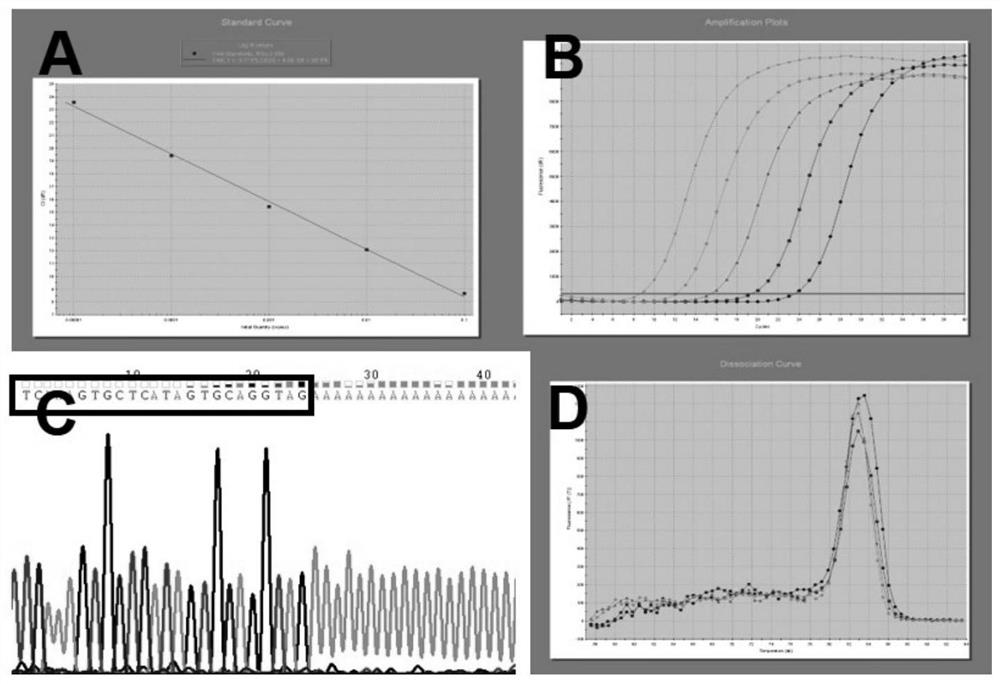
[0109] Example 1 Screening of miRNAs associated with confirmed HCV infection
[0110] 1.1 Collection of samples and collation of sample data
[0111] The inventors collected anti-HCV positive and HCV RNA positive blood donor samples at Shenzhen Blood Center from January to June 2014 (the samples used for research were collected, sampled, subpackaged, and kept under uniform conditions during the same period). By sorting out the sample data, the inventor selected 5 samples that were positive for HCV RNA and had an anti-HCV COI value greater than or equal to 3.8 for miRNA chip detection.
[0112] Among them, the statistics of anti-HCV positive blood donors in this study are shown in Table 7.
[0113] Table 7:
[0114]
[0115] 1.2 Isolation and purification of plasma miRNA
[0116] 1.2.1 Plasma preparation: 2ml of EDTA-anticoagulated peripheral venous blood was collected from all enrolled patients and healthy controls, and processed within 2 hours at room temperature. Bloo...
Embodiment 2
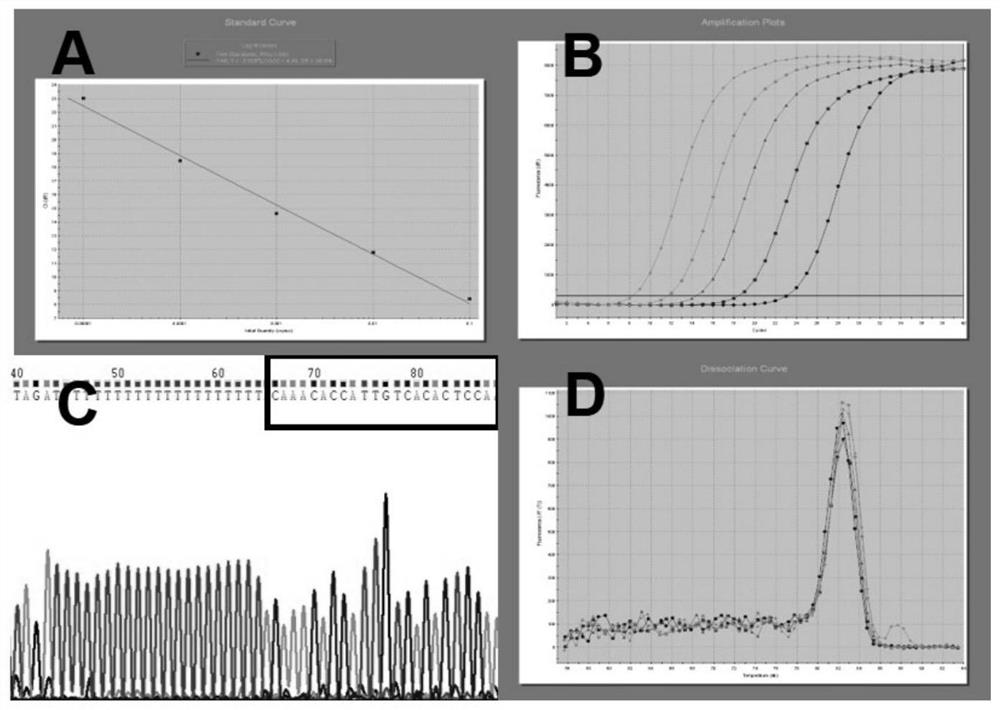
[0131] Embodiment 2QPCR verifies the miRNA of differential expression
[0132] According to the detection results of the miRNA chip, miRNA-20 and miRNA-122 were selected for large sample QPCR verification.
[0133] 133 anti-HCV positive samples and 56 healthy controls were selected according to the method of sample collection and sample data arrangement in Example 1.
[0134] 2.1 The RNA extraction process is the same as in Example 1.
[0135] 2.2 Reverse transcription of miRNA to synthesize cDNA:
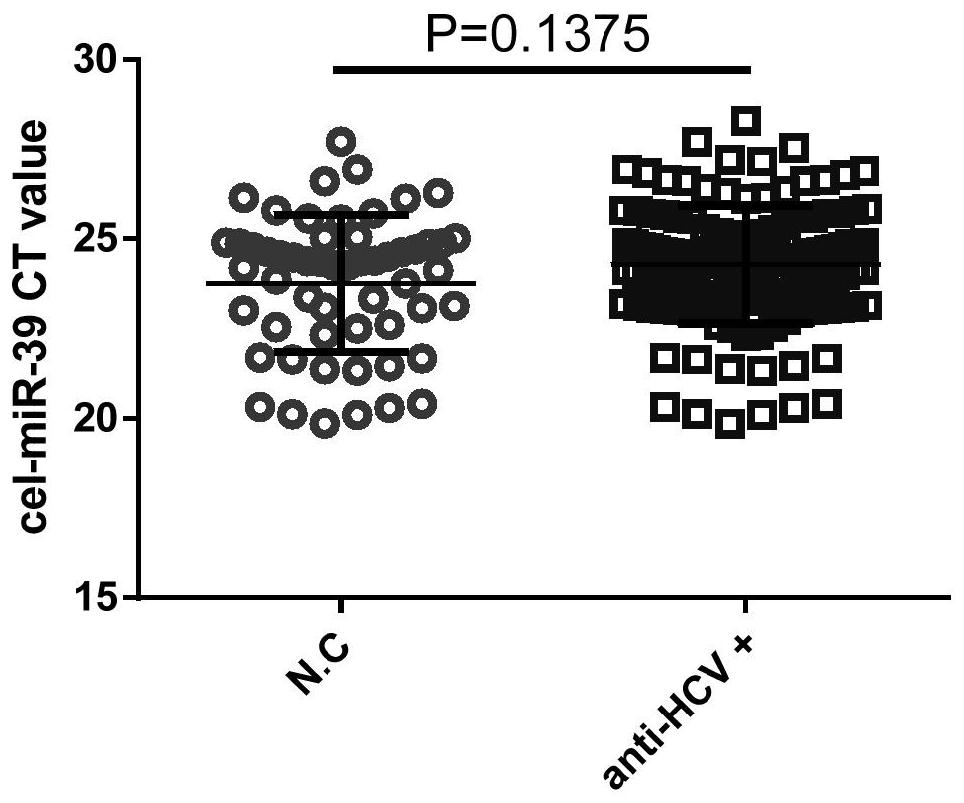
[0136] Each miRNA reverse transcription stem-loop primer is designed according to the specific sequence of the miRNA in miRBase, and the total RNA is reverse transcribed into the corresponding cDNA by a two-step method. The reverse transcription stem-loop primers designed respectively for miRNA-20 and miRNA-122 have the nucleotide sequences shown in SEQID NO:3,5. The inventor simultaneously performed QPCR for quantitative analysis of cel-miR-39 with no expression difference. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com