Preparation method of azodicarbonamide
A technology of azodicarbonamide and dimethylformyl, which is applied in the field of preparation of azodicarbonamide, can solve the problems of long steps and low production efficiency, and achieve the effect of less three wastes and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The preparation method according to the embodiment of the present invention comprises the following steps:
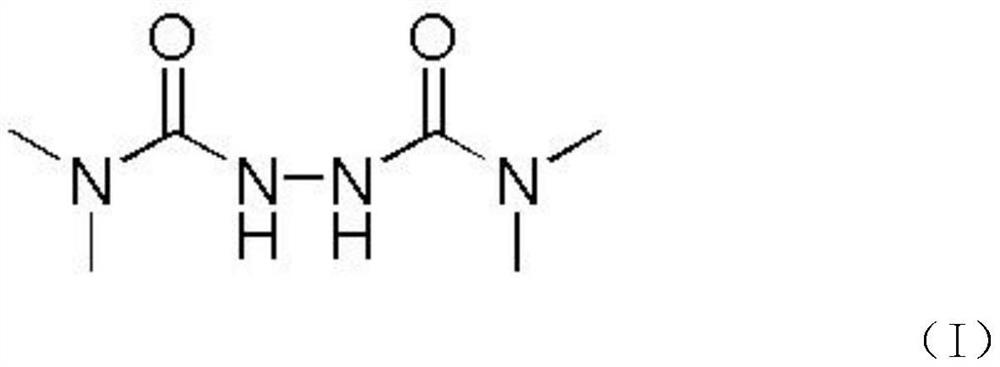
[0025] Step S1, reacting hydrazine hydrochloride and dimethyl formyl chloride to generate an intermediate, the chemical structural formula of the intermediate is shown in the following formula (I):
[0026]
[0027] That is to say, first, using hydrazine hydrochloride and dimethylformyl chloride as raw materials, the intermediate TMDA-1 having the chemical structural formula as shown in the above formula (I) is obtained.
[0028] Specifically, its chemical reaction formula is as shown in the following formula (1):
[0029]
[0030] Wherein, in order to promote the progress of the reaction, the hydrazine hydrochloride can be reacted with dimethylformyl chloride under the action of the first organic base in a tetrahydrofuran solution of hydrazine hydrochloride.
[0031] The existence of the first organic base removes hydrogen ions and chloride ions at the en...
Embodiment 1
[0051] (1) Preparation of compound TMAD-1
[0052]Take a 250mL reaction bottle and add 39.25g of hydrazine hydrochloride, 0.233mol, 2.5eq) and tetrahydrofuran (100mL, 5P), and add triethylamine (51.6g, 0.326mol, 3.5eq) dropwise in an ice-water bath with temperature controlled at 10-15°C, and continue to maintain Dimethylformyl chloride (10 g, 0.093 mol, 1.0 eq) was added dropwise at high temperature, and the reaction was completed after 4 hours of heat preservation. The reaction solution was washed with water (50mL*2), dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 13.1g of TMAD-1 with a yield of 81%.
[0053] (2) Preparation of compound TMAD
[0054] Add TMAD-1 (13.1g, 0.075mol, 1.0eq), NBS (13.4g, 0.075mmol, 1.0eq) into dichloromethane (100mL, 8P), add pyridine (5.9g , 0.075mol, 1.0eq), and incubated for 3 hours. The reaction solution was washed with 50 mL of water and 50 mL of saturated brine, dried over anhydrous sodium sulfate, filtered, and ...
Embodiment 2
[0058] (1) Preparation of compound TMAD-1
[0059] Take a 10L reaction bottle and add hydrazine hydrochloride (1.963kg, 28.65mol, 2.5eq) and tetrahydrofuran (5L, 5P), add triethylamine (2.58kg, 16.3mol, 3.5eq) dropwise in an ice-water bath with temperature controlled at 10-15°C, and continue to maintain Dimethylformyl chloride (500 g, 4.65 mol, 1.0 eq) was added dropwise at high temperature, and the reaction was completed after 6 hours of heat preservation. The reaction solution was washed with water (2.5L*2), dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 701g of TMAD-1 with a yield of 86.5%.
[0060] (2) Preparation of compound TMAD
[0061] Add TMAD-1 (701g, 4.02mol, 1.0eq), NBS (715.6g, 4.02mol, 1.0eq) into dichloromethane (5.6L, 8P), and add pyridine (317.6g , 4.02mol, 1.0eq), and incubated for 3 hours. The reaction solution was washed with 2L of water and 2L of saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com