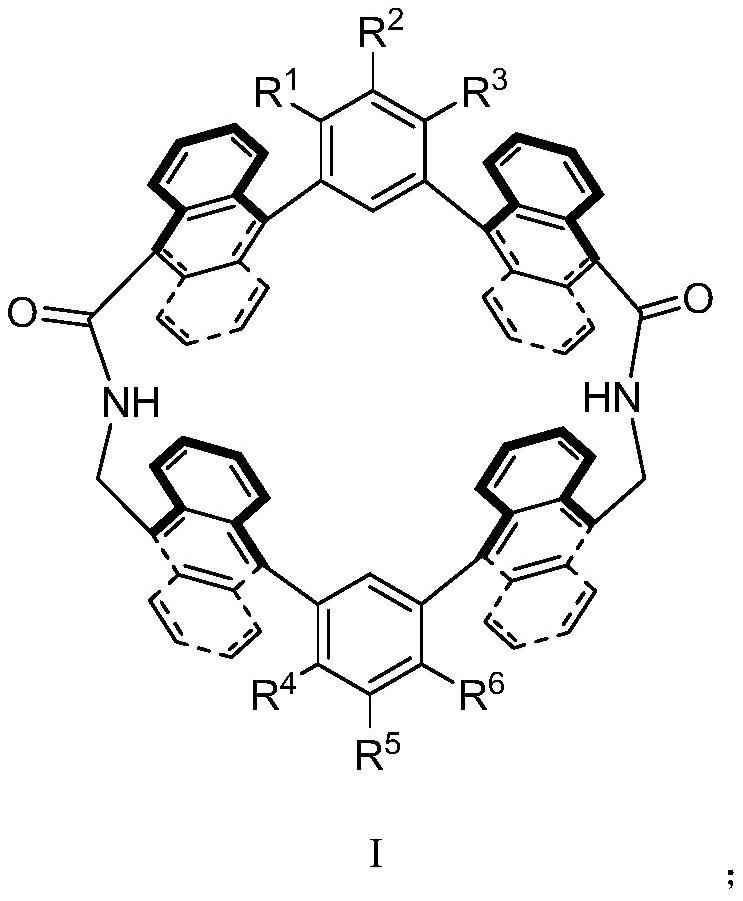
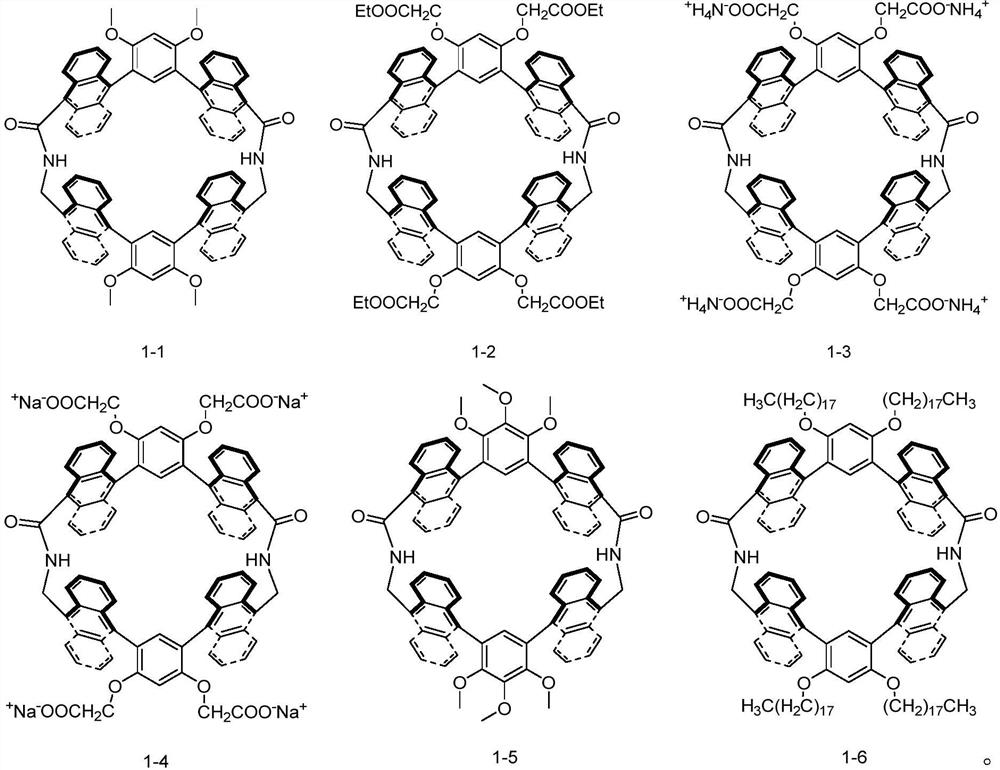
Anthryl macrocyclic molecule as well as preparation method and application thereof
A macrocyclic molecule, anthracenyl technology, applied in the field of anthracenyl macrocyclic molecules and its preparation, can solve the problems of lack of fluorescence properties, weakened non-covalent weak interaction strength between molecules, limited driving force, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
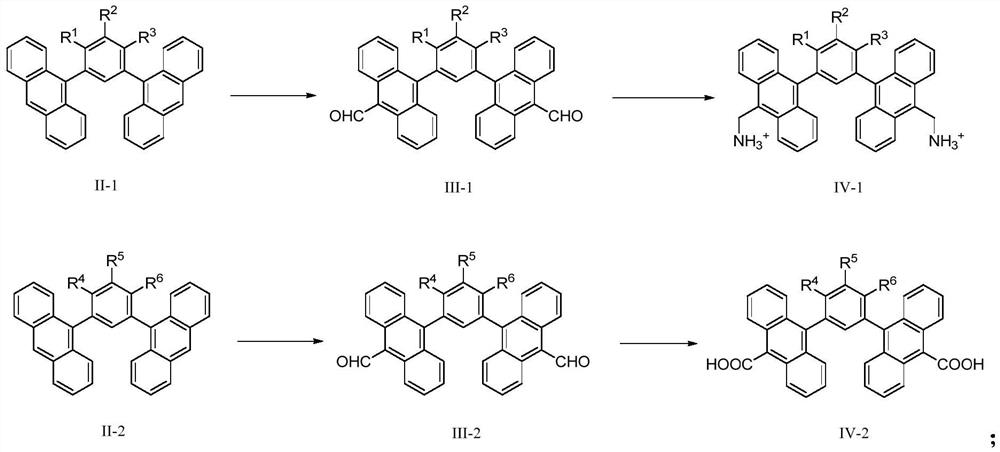
[0047] Another aspect of the present invention also provides the preparation method of the aforementioned anthracenyl macrocyclic molecule, which comprises the following steps:
[0048] Provide the rigid bridged bisanthracene shown in formula II-1, make it undergo acylation reaction, prepare the bisaldehyde group rigid bridged bisanthracene shown in formula III-1; the bisaldehyde shown in formula III-1 Rigid bridging bisanthracene undergoes an amination reaction to prepare a compound shown in formula IV-1;
[0049] Provide the rigid bridged bisanthracene shown in formula II-2, make it undergo acylation reaction, prepare the bisaldehyde group rigid bridged bisanthracene shown in formula III-2; the bisaldehyde shown in formula III-2 The base rigid bridging bisanthracene is through oxidation reaction, prepares the compound shown in formula IV-2;
[0050]
[0051] Carrying out cyclization reaction of compounds shown in IV-1 and IV-2 to prepare anthracenyl macrocyclic molecules...
Embodiment 1
[0077] (1) Preparation of bisaldehyde group rigid bridged bisanthracene compound A:
[0078]
[0079] Intermediate Compound S 0 Preparation: Dissolve 9-bromoanthracene (12g, 45mmol) in dry tetrahydrofuran (300mL), add n-butyl lithium in dry tetrahydrofuran (2.60M, 19.2mL, 50.6mmol ). After maintaining -80°C and stirring for two hours, zinc chloride (9.1 g, 66.7 mmol) was added. Maintain -80°C, stir for four hours, then warm up to room temperature, and stir for one day. 1,3-Dibromo-4,6-dimethoxybenzene (3.4g, 11.5mmol), bis(cyanobenzene)palladium dichloride (0.44g, 1.2mmol) and tri-tert-butylphosphine in n-hexyl Add alkane solution (10%wt, 5.6mL, 2.3mmol) into 50mL dry THF, and stir at room temperature for half an hour under nitrogen protection. The solution was transferred and injected into the reaction system which had been stirred at room temperature for one day, and then the temperature was raised to 85° C. and stirred for two days. Afterwards, the reaction was cool...
Embodiment 2
[0091] (1) Preparation of bisaldehyde group rigid bridged bisanthracene compound B:
[0092]
[0093] Synthetic intermediate product S 1 : Compound S 0 (2.4g, 5.0mmol) was dissolved in 50mL of dichloromethane, BBr was added at 0°C 3 (5.0 mL, 50.0 mmol). After stirring at 0°C for half an hour, the mixture was warmed up to room temperature and stirred for four hours. Pour the reaction solution into an ice-water bath, adjust the pH to 5-6 with NaOH solution, and extract with dichloromethane (50 mL×3) and ethyl acetate (50 mL×3). The organic phases were combined, and the organic solvent was removed under reduced pressure to obtain the solid product S 1 (2.9g, 90%).
[0094] The preparation of compound B: intermediate product S 1 (6.3g, 10.0mmol) was dissolved in 200mL of dichloromethane, and 1,1-dichloromethyl ether (3.6mL, 40.0mmol) was added. The temperature was lowered to 0°C, and TiCl was added dropwise under stirring 4 (4.0 mL, 40.0 mmol). Continue to maintain 0° C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com