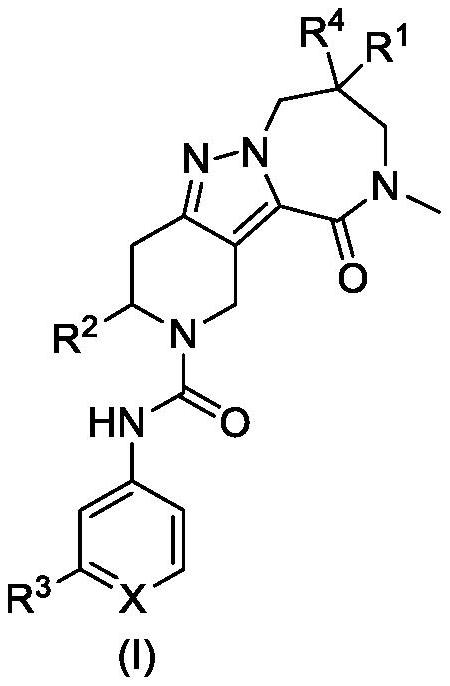
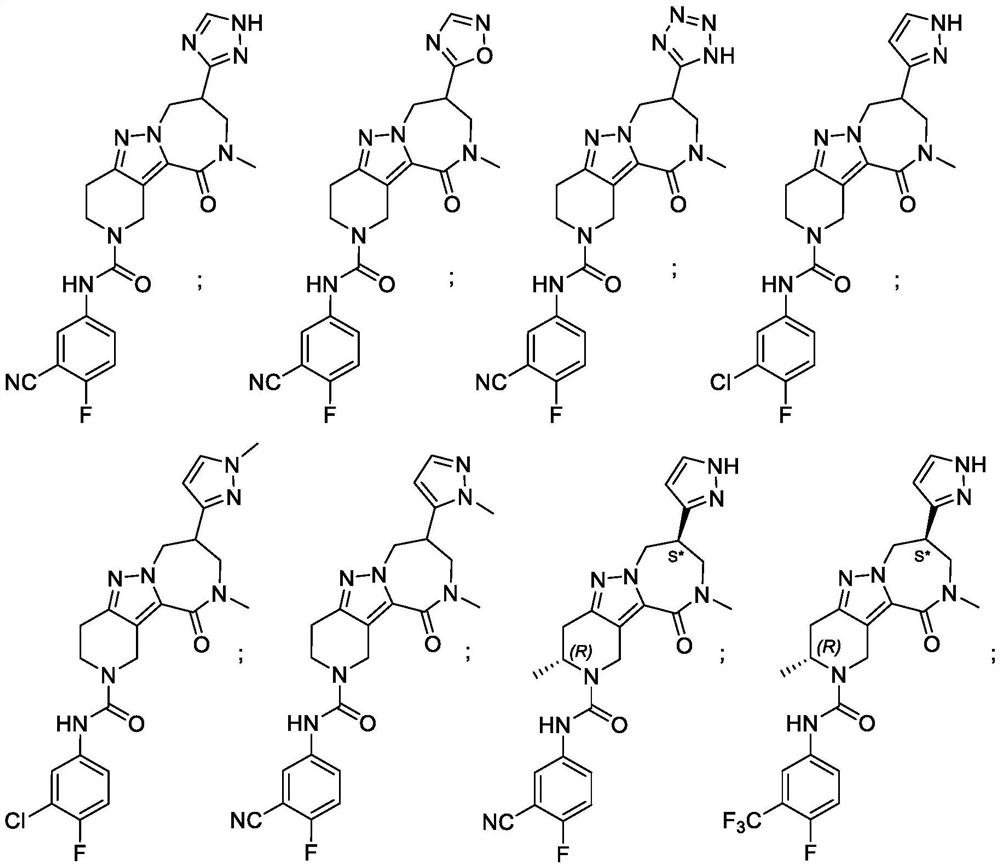
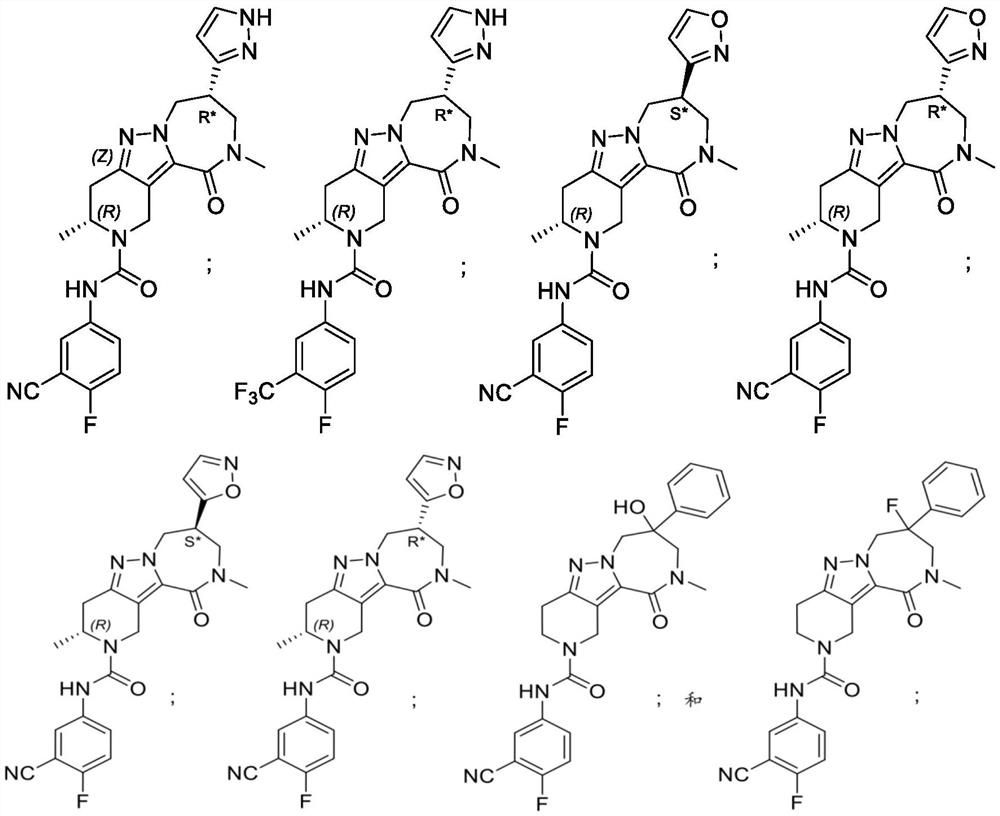
Diazepinone derivatives as capsid assembly modulators
A compound, heteroaryl technology, applied in the field of novel diazepine compounds, can solve problems such as difficult selection of resistance selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
[0169] By the first example of the substituent, if the substituent S 1 实例 Be s 1 S 2 One of them, and substituent S 2 实例 Be s 3 S 4 One of them, these assignments refer to embodiments of the disclosure given in accordance with the following: s 1 实例 Be s 1 And s 2 实例 Be s 3 S 1 实例 Be s 1 And s 2 实例 Be s 4 S 1 实例 Be s 2 And s 2 实例 Be s 3 S 1 实例 Be s 2 And s 2 实例 Be s 4 And the equivalents of each of this type of selection. For the sake of simplicity, this paper uses shorter terms accordingly "S 1 实例 Be s 1 S 2 One of them, and s 2 实例 Be s 3 S 4 One ", but not by way of limitation. In general terms, according to the above example on substituent terminology Zi are intended to illustrate various substituent groups herein according to the assignment. Forth herein for the above specification extends to substituents such as R when applicable 1 R 2 R 3 R 4 , PG and X, and any other generic substituent used herein is a member of group symbols.
[0170] Further, when more than on...
example
[0195] An exemplary compound which can be used in the present disclosure is now described with reference to the illustrative synthesis schemes used in the general preparations thereof and subsequent specific examples. The skilled person will recognize that in order to obtain a variety of compounds herein, the starting material can be appropriately selected such that the final desired substituent will be carried in or without suitable protection by a reaction scheme to produce a desired product. Alternatively, it may be necessary or desirable to use a suitable group instead of the final desired substituent, the suitable group can be carried by the reaction scheme and is replaced by the desired substituent. Unless otherwise stated, the variables are as defined above with reference formula (I) above. The reaction can be carried out between the melting point of the solvent and the reflux temperature, preferably between the reflux temperature of 0 ° C and the solvent. Conventional heat...
example 1
[0345] Example 1: N- (3-cyano-4-fluorophenyl) -10-methyl-11-oxo-8- (1H-1, 2, 4-triazole-3-yl) -3, 4 , 8,9,10,11-hexapyridine - pyridine and [4 ', 3': 3, 4] pyrazole [1,5-A] [1,4] Diazazhi-2 (7h) - formamide.
[0346]
[0347] Step A.10-methyl-8- (1H-1, 2, 4-triazole-3-yl) -3, 4, 7, 8, 9, 10-hexahydro-1H-pyridine [4 ', 3 ': 3,4] Pyrazole [1,5-A] [1,4] Diazazepo - 11 (2H) - ketone.
[0348] The 10-methyl-11-oxo -8- (1H-1,2,4- triazol-3-yl) -1,3,4,7,8,9- hexahydro-pyrido [2,3- ] pyrazolo [2,4-b] [1,4] diazepine-2-carboxylate (intermediate 2,40.00mg, 103.24μmol) solution was added TFA (3.00 mL) in DCM, (770.00mg, 6.75mmol, 500.00μL). The reaction mixture was stirred for 30min at 15 ℃. The mixture was concentrated under reduced pressure to afford the title compound as a yellow oil (40.00 mg, crude, TFA salt), which was used directly without purification in the next step.
[0349] Step B.N- (3-cyano-4-fluorophenyl) -10-methyl-11-oxo-8- (1H-1, 2, 4-triazole-3-yl) -3, 4, 8,9,10,11-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com