Application of pharmaceutical composition containing anti-PD-1 antibody in preparation of drugs for treating advanced non-small cell lung cancer
A non-small cell lung cancer, PD-1 technology, applied in the medical field, can solve the problems of EGFR mutant NSCLC patients not showing survival benefit and high incidence of interstitial pneumonia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: Clinical study of anti-PD-1 antibody combined with chemotherapy in the treatment of non-small cell lung cancer
[0109] Inclusion criteria: Eligible subjects must be (1) aged 18-75, (2) suffering from advanced or recurrent non-small cell lung cancer with EGFR sensitive mutations, (3) prior first-line EGFR-TKI treatment Failure, (4) no EGFR T790M mutation, 5) ECOG score 0 or 1, (6) no history of autoimmune disease, (7) no prior anti-PD-1 / or anti-PD-L1 immunity therapy.
[0110] Subjects must have evaluable lesions according to RECIST v1.1 criteria, no combined small cell lung cancer or squamous cell carcinoma, no other mutations that can be used for targeted therapy, no previous systemic chemotherapy, and no acceptance Long-term systemic immunosuppressive therapy.
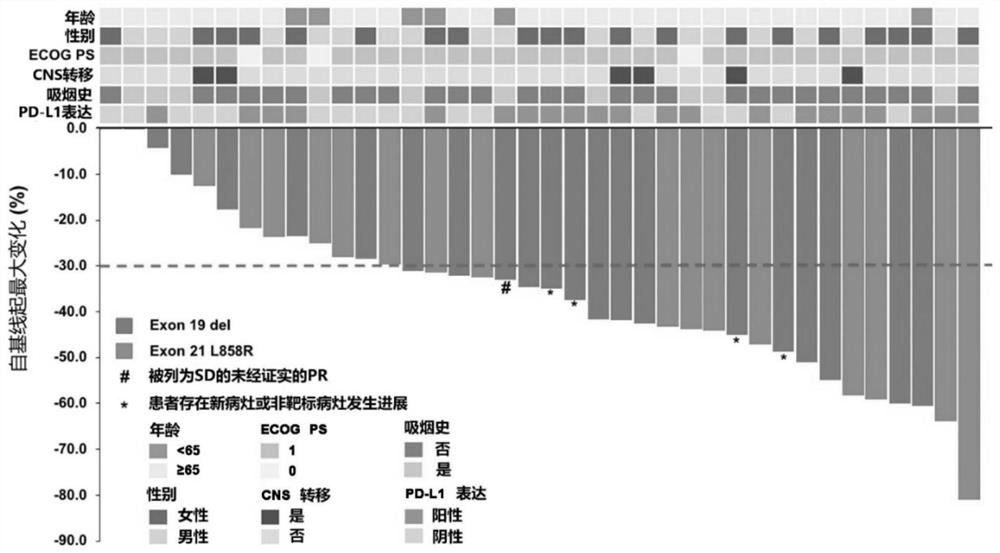
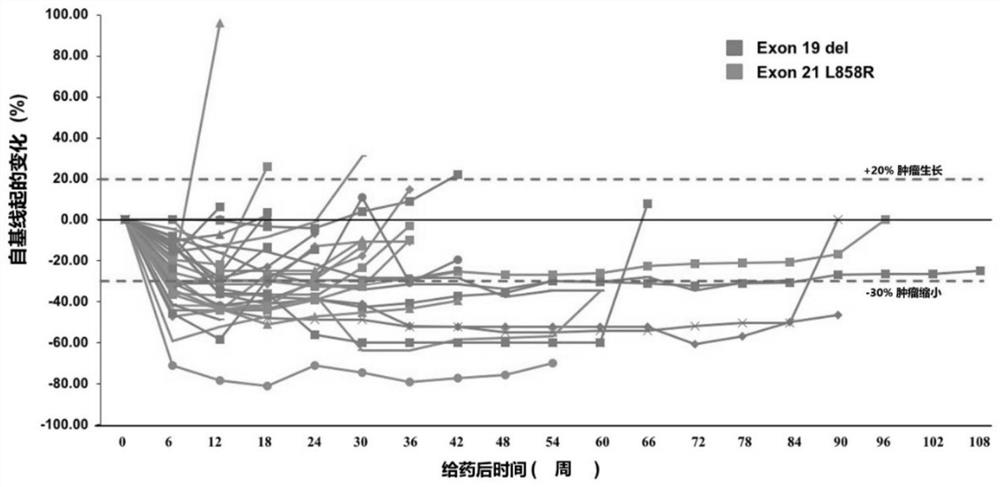
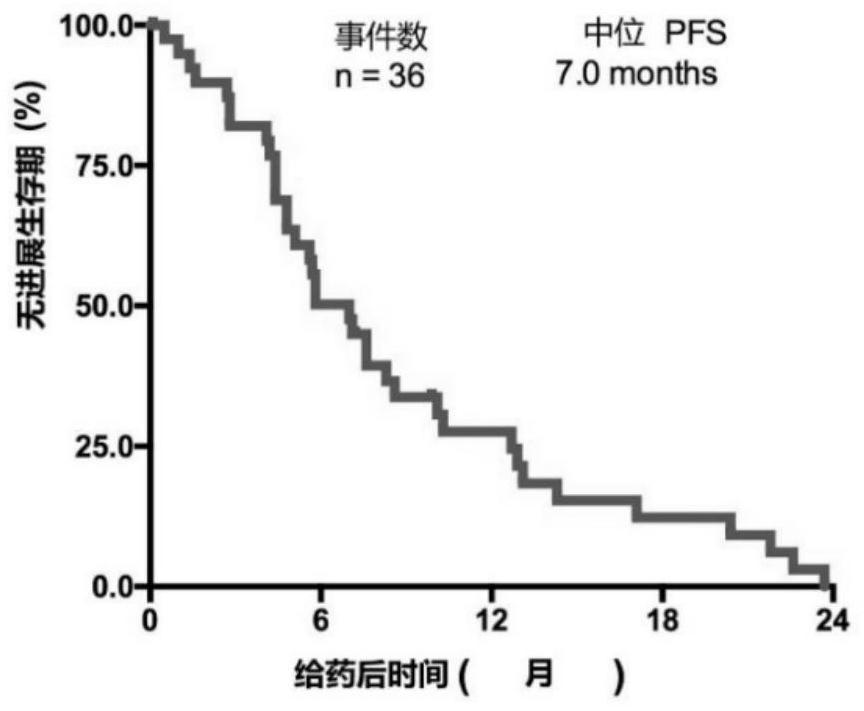
[0111] From April 2018 to March 2019, 65 patients with EGFR+ NSCLC were screened, and a total of 40 patients were included in this study. The median age of the subjects was 58 years (range: 19 ...
Embodiment 2
[0127] Example 2: Research on the correlation between biomarkers and clinical efficacy
[0128]2.1 WES
[0129] Of the 40 patients enrolled, we performed whole exome sequencing (WES) on tumor biopsies and paired peripheral blood from 34 of the patients. WES identified 7048 gene alterations, including 3505 missense mutations, 84 gene deletions, 123 rearrangements, 119 splice site substitutions, 349 truncations, and 2748 gene amplifications. Noted a significant difference in genetic alterations between partial response (PR) patients and non-PR patients ( Figure 2a ), we reasoned that genetic mutations might be used as predictive markers for this scheme. Genomic predictions of the top 100 most frequently mutated genes in relation to efficacy were performed using Maftools::survGroup (v2.6.05). Considering the small data set, we set a relatively strict criterion (P≤0.01) to screen genes or gene combinations with predictive ability. We found that the predictive model works well...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com