Preparation method of exosome, exosome prepared by preparation method and application of exosome
An exosome and autologous technology, applied in the preparation of exosomes, in the field of exosomes, to achieve the effect of promoting wound healing, reducing melanosis, and reducing traces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
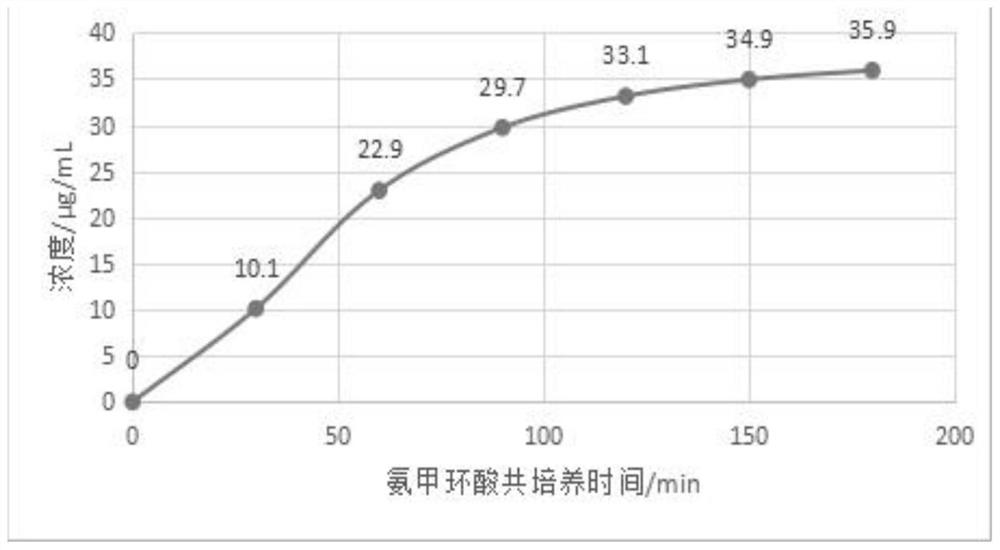
Image
Examples
Embodiment 1
[0065] This embodiment provides an exosome gel, which is prepared by the following preparation method:
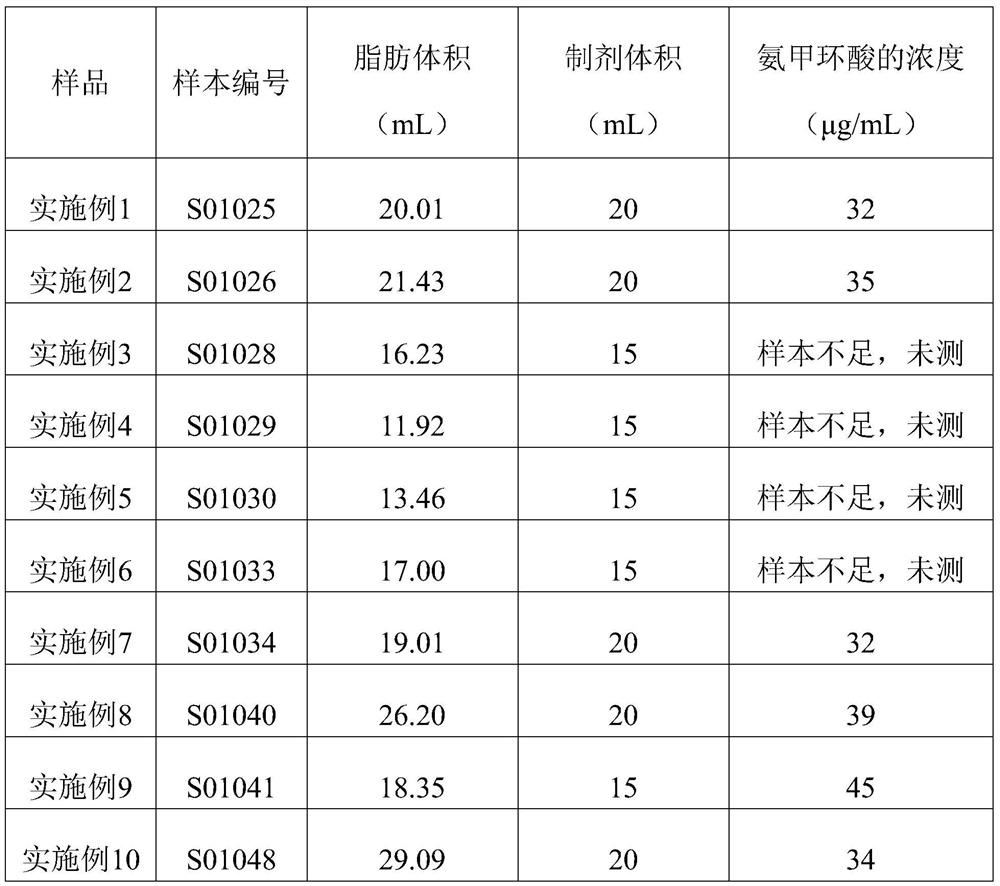
[0066] (1) Take 20.01 mL of autologous abdominal adipose tissue (sample number S01025) and digest it with 20 mL of trypsin digestion solution, digest it at 37°C for 10 minutes, gently shake the digested cell suspension, and transfer it to a centrifuge tube; Digest again with 15mL of trypsin digestion solution, digest at 37°C for 5min; shake the digested suspension gently, transfer to a centrifuge tube, and collect the digested cell suspension;
[0067] Wherein, the trypsin digestion liquid includes: 0.1% trypsin, 0.1 mM EDTA solution with a pH of 8.0 as a solvent;
[0068] Centrifuge the collected digested cell suspension at 1000rpm for 3min, discard the supernatant; add 10mL of PBS buffer, shake and wash, then centrifuge at 1000rpm for 3min, discard the supernatant; repeat the washing step once to obtain adipocytes;
[0069] (2) The digested and cleaned adipocytes were cu...
Embodiment 2
[0075] This embodiment provides an exosome gel, which is prepared by the following preparation method:
[0076] (1) Take 21.43 mL of autologous abdominal adipose tissue (sample number S01026) and digest it with 20 mL of trypsin digestion solution, digest it at 37°C for 10 minutes, gently shake the digested cell suspension, and transfer it to a centrifuge tube; Digest again with 15mL of trypsin digestion solution, digest at 37°C for 5min; shake the digested suspension gently, transfer to a centrifuge tube, and collect the digested cell suspension;
[0077] Wherein, the trypsin digestion liquid includes: 0.1% trypsin, 0.1 mM EDTA solution with a pH of 8.0 as a solvent;
[0078] Centrifuge the collected digested cell suspension at 1000rpm for 3min, discard the supernatant; add 10mL of PBS buffer, shake and wash, then centrifuge at 1000rpm for 3min, discard the supernatant; repeat the washing step once to obtain adipocytes;
[0079] (2) The digested and cleaned adipocytes were cu...
Embodiment 3
[0085]This embodiment provides an exosome gel, which is prepared by the following preparation method:
[0086] (1) Take 16.23 mL of autologous abdominal adipose tissue (sample number S01028) and digest it with 15 mL of trypsin digestion solution, digest it at 37°C for 10 minutes, gently shake the digested cell suspension, and transfer it to a centrifuge tube; Digest again with 15mL of trypsin digestion solution, digest at 37°C for 5min; shake the digested suspension gently, transfer to a centrifuge tube, and collect the digested cell suspension;
[0087] Wherein, the trypsin digestion liquid includes: 0.1% trypsin, 0.1 mM EDTA solution with a pH of 8.0 as a solvent;
[0088] Centrifuge the collected digested cell suspension at 1000rpm for 3min, discard the supernatant; add 10mL of PBS buffer, shake and wash, then centrifuge at 1000rpm for 3min, discard the supernatant; repeat the washing step once to obtain adipocytes;
[0089] (2) The digested and cleaned adipocytes were cul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com