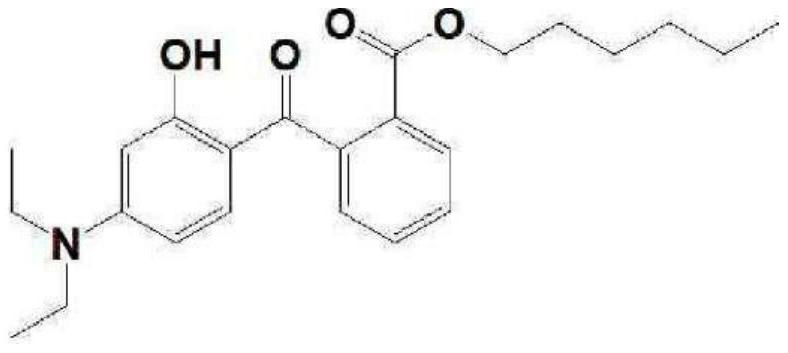
Method for preparing diethylamino hydroxybenzoyl hexyl benzoate
A technology of diethylamino hydroxybenzoyl benzoic acid and hydroxybenzoyl, which is applied in the field of manufacturing diethylamino hydroxybenzoyl hexyl benzoate, can solve problems such as difficulty in application and mass production constraints, and achieve mass production Effects of production, high yield, and reduced number of purifications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
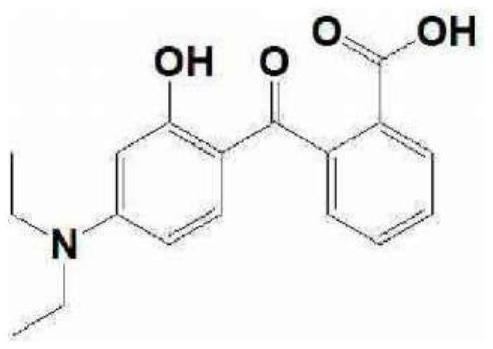
[0061] Production Example 1. Synthesis of 2-(4-N,N-diethylamino-2-hydroxybenzoyl)benzoic acid
[0062] After putting 3-diethylaminophenol (1.0 kg, 7.87 mol) and phthalic anhydride (1.17 kg, 7.87 mol) into the 3-neck flask, it was stirred with toluene (5.0 L). After raising the internal temperature of the reactor to 110-115° C. and stirring for 2 hours, it was cooled to 0-10° C. and the precipitated solid was filtered to obtain the title compound (1.7 kg, 89.9%).
[0063] 1 H NMR (CDCl 3 ): 12.52(s,1H),7.91(dd,1H),7.62(m,2H),7.33(dd,1H),6.74(d,1H),6.13(dd,1H),6.20(d,1H) ,1.16(m,6H)
manufacture example 2-11
[0064] Production Example 2-1. Synthesis of 1-chlorohexane
[0065] 1-Hexanol (1.2kg, 11.70mol) and dimethylformamide (DMF, 8.5g, 0.12mol) were stirred and SOCl was added dropwise while maintaining the internal temperature below 30°C 2 (2.1 kg, 17.6 mol). After completion of the dropwise addition, the internal temperature was raised to 80 to 90° C. and stirred for 5 hours. After confirming the completion of the reaction, cooling was performed, and 5 L of purified water was added to separate layers to obtain the title compound (1.3 kg, 94.2%).
[0066] 1 H NMR (CDCl 3 ): 3.49(t,2H), 1.72(m,2H), 1.28(m,2H), 1.27(m,4H), 0.88(m,3H).
manufacture example 2-21
[0067] Production Example 2-2. Synthesis of 1-bromohexane
[0068] 1-Hexanol (1.2 kg, 11.70 mol) and tetrahydrofuran (THF, 6.0 L) were stirred, and PBr was added dropwise while maintaining the internal temperature below 10 °C 3 (3.2 kg, 17.6 mol). After completion of the dropwise addition, the internal temperature was maintained at 10° C. or lower and stirred for 3 hours. After confirming the completion of the reaction, 5 L of purified water was thrown in and the layer was separated to obtain the title compound (1.8 kg, 91.4%).
[0069] 1 H NMR (CDCI 3 ): 3.40(t,2H), 1.85(m,2H), 1.43(m,2H), 1.31(m,4H), 0.90(m,3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bulk density | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com