Method for manufacturing lithium-ion-conducting glass ceramic
一种制造方法、玻璃陶瓷的技术,应用在玻璃制造设备、玻璃的成型、制造工具等方向,能够解决锂离子传导性不太良好等问题,达到改善离子传导性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
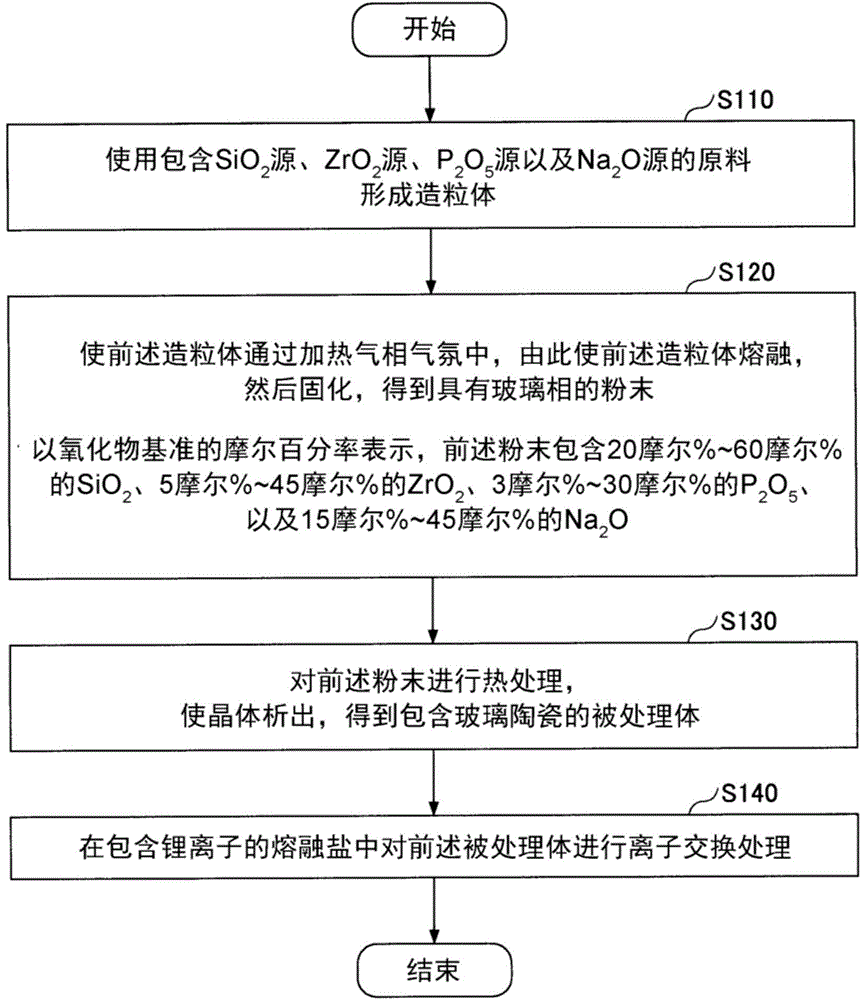
[0083] (Preparation of Raw Material Slurry)
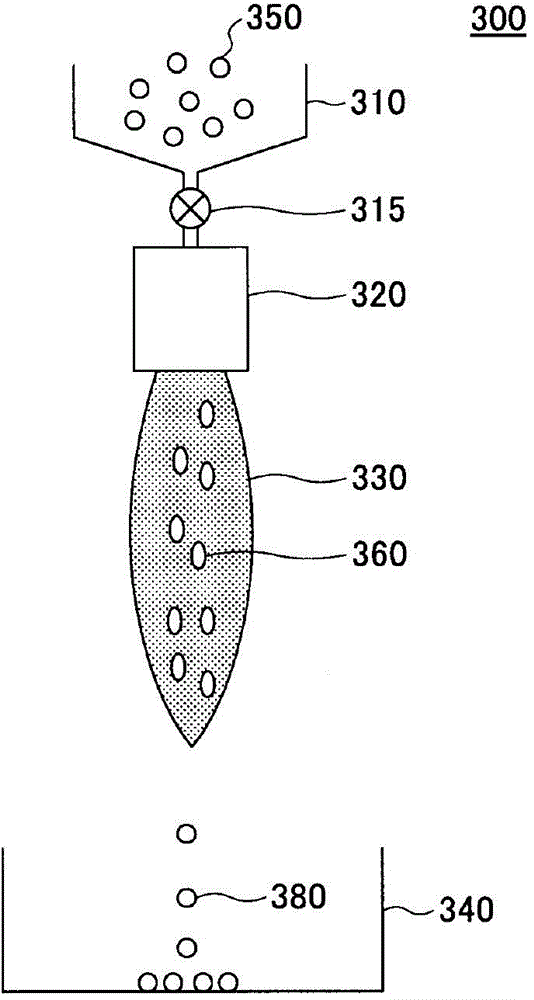
[0084] When forming granules by the spray drying method, first, raw material slurry is prepared using the raw material prepared by the above-mentioned method.
[0085] The solvent used for the slurry is not particularly limited, and the solvent may be, for example, water, ethanol, acetone, toluene, and / or hexane. Among them, when using an alcohol-based solvent, it is necessary to use a spray drying device with an explosion-proof device, so water is preferably used from the viewpoint of operational efficiency.
[0086] Raw material slurry is obtained by adding and mixing prepared raw materials to a solvent. In addition, what is necessary is just to dissolve a part of said raw material in a solvent. Moreover, the mixing ratio of a raw material and a solvent is not specifically limited. The ratio of the solvent may be, for example, in the range of 40% by weight to 60% by weight relative to the entire raw material slurry.
[0087] ...
example 1
[0133] (Preparation of samples for evaluation)
[0134] The evaluation sample of Example 1 was produced by the following method, and its characteristic was evaluated.
[0135] Samples for evaluation were produced in the following procedure.
[0136] First, mix silica sand (average particle size 30 μm), zirconia (ZrO 2 ) powder (average particle size 20μm), ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) powder (average particle size 20μm) and sodium carbonate (Na 2 CO 3 ) powder (average particle size 30μm), making SiO 2 -ZrO 2 -P 2 o 5 -Na 2 O conversion was calculated as the raw material composition shown in the column of Example 1 of Table 1, and the powder mixed raw material was prepared. The particle size of each compound is a value measured by a laser diffraction scattering type dry particle size measuring device.
[0137] Next, 2 kg of the powder mixed raw material was added to 2 liters of distilled water and mixed to prepare a raw material slurry. Furthermo...
example 2
[0165] By the same method as in Example 1, the sample for evaluation of Example 2 was produced, and the characteristic was evaluated.
[0166] where, in this Example 2, as P 2 o 5 Source, using sodium hexametaphosphate (NaPO 3 ) 6 Powder (average particle size 150μm) instead of ammonium dihydrogen phosphate (NH 4 h 2 PO 4 )powder.
[0167] It should be noted that, also in Example 2, gas was generated during the grinding process, and the pressure in the container increased. Therefore, degassing was performed every 5 minutes. However, ammonia odor was not confirmed. The pH of the obtained raw material slurry was 8.3. The obtained granules had a particle diameter of about 50 μm. The porosity of the granules is in the range of 30% to 80%. Other conditions are the same as in Example 1.
[0168] Using the sample for evaluation of Example 2, the ion conductivity was measured by the method described above. As a result of the evaluation, the ion conductivity of the evaluat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| apparent porosity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com