Method for preparing leuprorelin by combining solid phase and liquid phase
A technology of leuprolide and liquid phase, which is applied in the field of preparing leuprolide by combining solid phase and liquid phase, can solve the problems of inconvenient and difficult large-scale production, achieve high synthesis yield and avoid product instability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
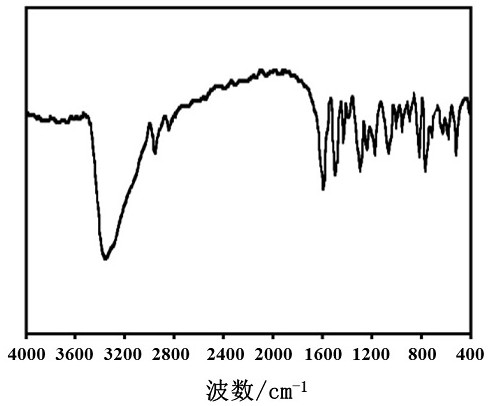
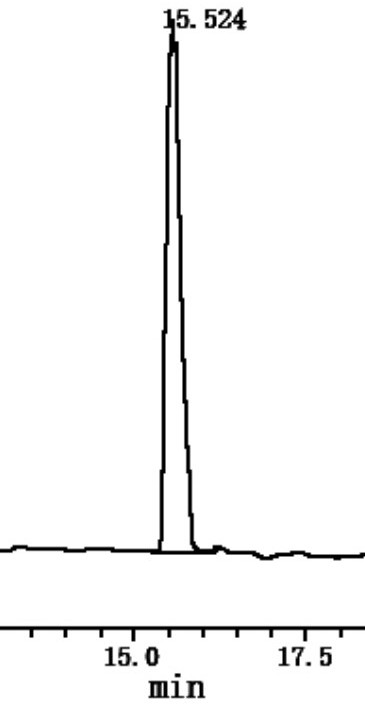
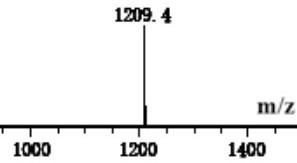
Image
Examples
Embodiment 1
[0078] A method for preparing leuprolide by combining solid phase and liquid phase,
[0079] Preparation of the liquid phase carrier: Add potassium carbonate to DMF, then add 3-ethoxy-4-hydroxybenzaldehyde and 2-(2-ethoxyphenoxy)bromoethane, protect with nitrogen, at 70°C Stir the reaction for 24 h at a temperature of 100 °C, add deionized water to precipitate, filter, wash, and dry to obtain benzaldehyde compounds; under nitrogen protection, add benzaldehyde compounds into the tetrahydrofuran solution, add sodium borohydride, The reaction was stirred at high temperature for 6 h. After the reaction was completed, deionized water was added to the solution to precipitate a precipitate, which was filtered, washed, and dried to obtain a liquid phase carrier. The usage amount of potassium carbonate is 1.2 wt% of DMF, the addition amount of 3-ethoxy-4-hydroxybenzaldehyde is 6 wt% of DMF, the addition of 2-(2-ethoxyphenoxy)bromoethane The amount is 9 wt% of DMF, the tetrahydrofuran ...
Embodiment 2
[0094] A method for preparing leuprolide by combining solid phase and liquid phase,
[0095] Preparation of the liquid phase carrier: Add potassium carbonate to DMF, then add 3-ethoxy-4-hydroxybenzaldehyde and 2-(2-ethoxyphenoxy)bromoethane, protect with nitrogen, at 70°C Stir the reaction for 24 h at a temperature of 100 °C, add deionized water to precipitate, filter, wash, and dry to obtain benzaldehyde compounds; under nitrogen protection, add benzaldehyde compounds into the tetrahydrofuran solution, add sodium borohydride, The reaction was stirred at high temperature for 6 h. After the reaction was completed, deionized water was added to the solution to precipitate a precipitate, which was filtered, washed, and dried to obtain a liquid phase carrier. The usage amount of potassium carbonate is 1.2 wt% of DMF, the addition amount of 3-ethoxy-4-hydroxybenzaldehyde is 6 wt% of DMF, the addition of 2-(2-ethoxyphenoxy)bromoethane The amount is 9 wt% of DMF, the tetrahydrofuran ...
Embodiment 3
[0110] A method for preparing leuprolide by combining solid phase and liquid phase,
[0111] Compared with Example 2, the present embodiment is only different in that in the preparation of Fmoc-Arg(Pbf)-Pro-Wang resin, the usage amount of 2-[(3-aminophenyl)sulfonyl)ethanol is DMF 1.9 wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com