A kind of sitagliptin chiral intermediate and asymmetric synthesis method
A chiral intermediate, sitagliptin technology, applied in the field of pharmaceutical preparation, can solve the problems of rare raw materials, high cost, expensive and the like, and achieves the effects of mild reaction conditions, high dr ratio and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
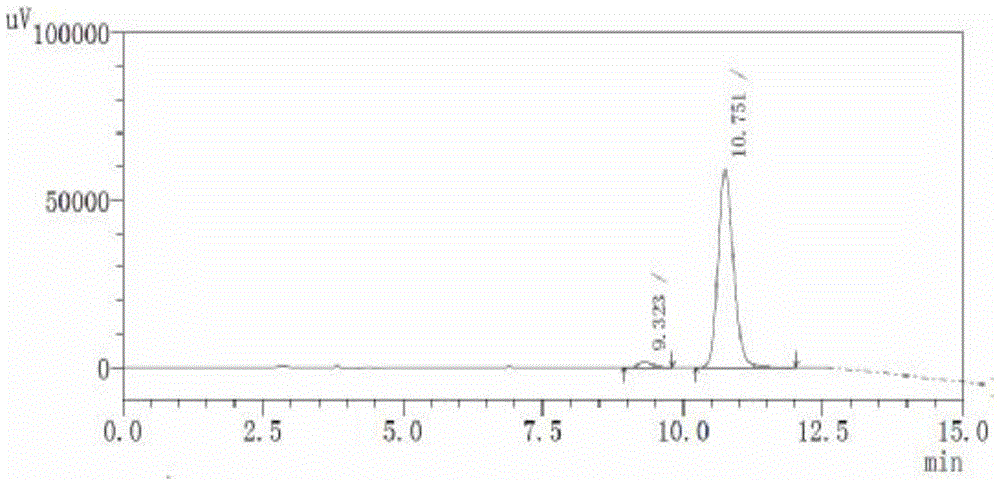
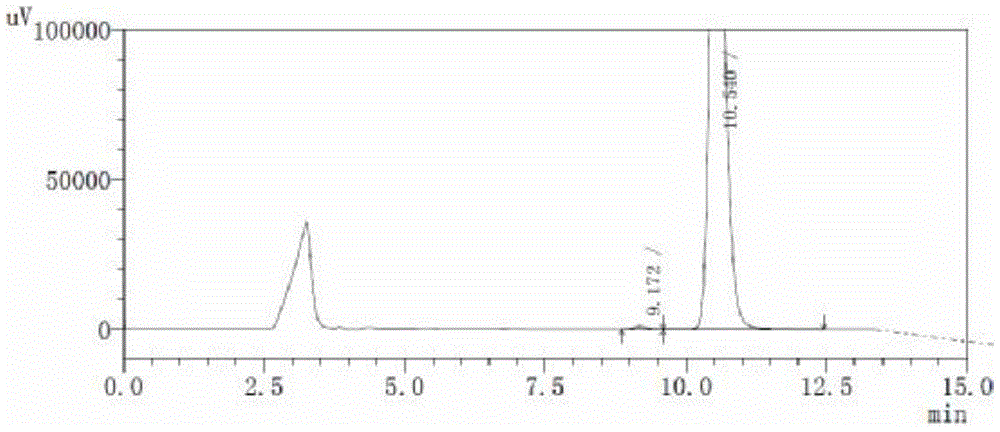
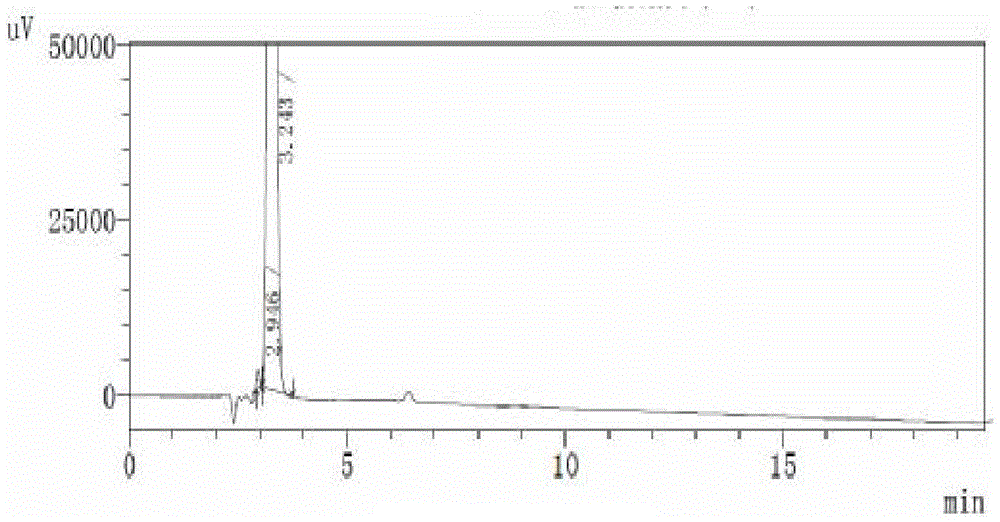
Image
Examples
Embodiment 1
[0044] A kind of asymmetric synthetic method of sitagliptin chiral intermediate, comprises the following steps:
[0045] Step 1: Using 2,4,5-trifluorophenylacetic acid as the starting material, obtain 2-(2,4,5-trifluorophenyl)ethanol through reduction reaction, the reaction formula is:
[0046]
[0047] The reducing agent in this step selects NaBH for use 4 、CH 3 SO 3 H;
[0048] Specific steps:
[0049] Add NaBH to the 250ml three-necked bottle 4 (12 grams), THF (tetrahydrofuran) 160ml, ice bath, when the temperature drops to -15 ~ 5 ℃, slowly add CH diluted with 10ml THF 3 SO 3 H, control the rate of addition so that the temperature is below -15-10°C, and the addition is complete within 40 minutes. After stirring for 20 minutes to 200 minutes below -15-10°C, add dropwise A dissolved in 25 minutes, control the rate of addition, keep the temperature below -15-10°C, add within 30 minutes to 90 minutes, and raise the reaction to Room temperature, after 1 hour to 5 hou...
Embodiment 2
[0063] The present embodiment is based on embodiment 1, and A and NaBH in step 1 in embodiment 1 4 、CH 3 SO 3 The molar ratio of H is set to: 1:1:6, namely adding NaBH 4 (12g), THF (tetrahydrofuran) (160ml), CH 3 SO 3 H (16ml); set the molar ratio of B to IBX and (R)-(+)-tert-butylsulfinamide in step 2 to 1:3:1.1, that is, add B (7.5 grams), IBX (71 grams ), (R)-(+)-tert-butylsulfinamide (22.7 grams); C in step 3 was mixed with dimethyl malonate, NaHCO 3 The molar ratio is set to 1:1.3:4, namely C (4.5 grams), dimethyl malonate (2.5ml), NaHCO 3 (10.8 grams).
Embodiment 3
[0065] The present embodiment is based on embodiment 1, and A and NaBH in step 1 in embodiment 1 4 、CH 3 SO 3 The molar ratio of H is set to: 1:1:1, namely adding NaBH 4 (10 grams), THF (tetrahydrofuran) (80ml), CH 3 SO 3 H (2.65ml); set the molar ratio of B to IBX and (R)-(+)-tert-butylsulfinamide in step 2 to 1:1:1.1, that is, add B (7.5 grams), IBX (6.1 gram), (R)-(+)-tert-butylsulfinamide (5.6 grams); C in step 3 was mixed with dimethyl malonate, NaHCO 3 The molar ratio is set to 1:1:1.5, namely C (9 grams), dimethyl malonate (4.1ml), NaHCO 3 (4.05 grams).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com