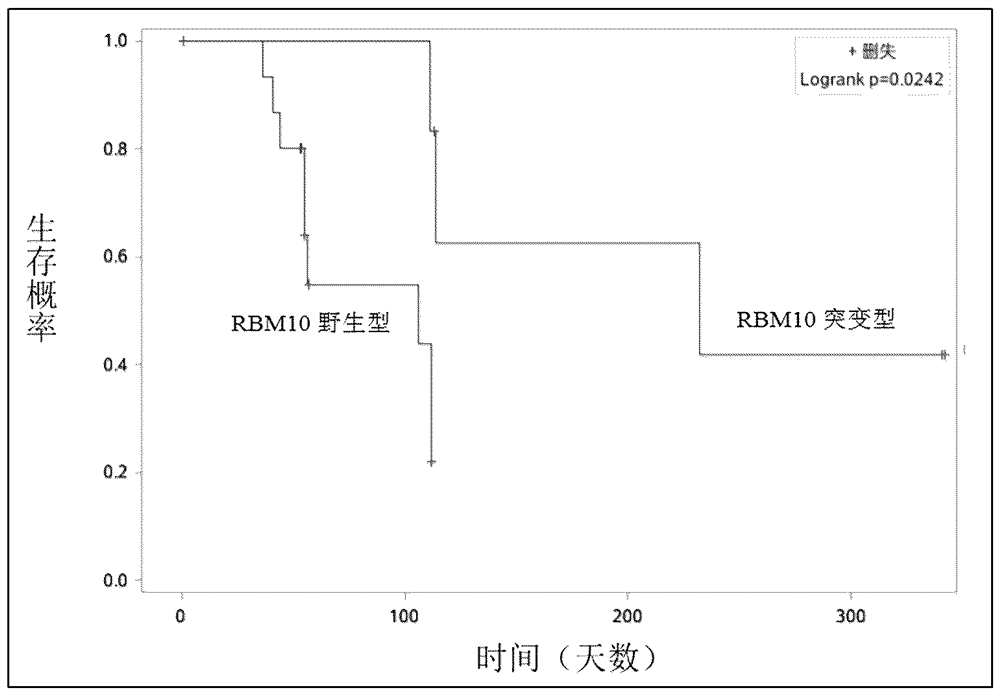
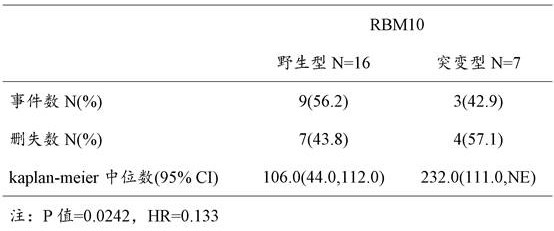
Application of RBM10 gene
A gene and application technology, applied in the application field of RBM10 gene, can solve the problems such as not getting significant benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Phase Ib clinical trial of Cioronib monotherapy for relapsed and refractory ovarian cancer
[0060] Test drug: Cioroni capsules, specifications: 5mg, 25mg. Produced by Shenzhen Microchip Biotechnology Co., Ltd.
[0061] Dosing regimen: Cioroni capsules are administered at 50 mg / day, QD (no adjustment for body weight or body surface area). Take every morning on an empty stomach with water, swallowing the whole capsule whole. Continuous administration for 28 days constitutes a treatment cycle, and there is no interval between each treatment cycle.
[0062] Number of cases: 25 cases were enrolled, of which 23 cases had curative effect evaluation.
[0063] standard constrain:
[0064] 1. Age ≥ 18 years old, ≤ 70 years old, female;
[0065] 2. Epithelial ovarian cancer, fallopian tube cancer or primary peritoneal cancer confirmed by histology;
[0066] 3. Subjects need to have received platinum-based chemotherapy regimens, that is,
[0067] a) Platinum-resi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com