Application of tanshinone IIA sulfonatesodium in preparation of medicine
A technology of sodium sulfonate and tanshinone, which is applied in the directions of drug combination, pharmaceutical formula, medical preparation containing active ingredients, etc., can solve problems such as single species
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Trial grouping and intervention
[0023] Sham operation group (SHAM group): 10 rats
[0024] Experimental group (ligation of the left anterior descending coronary artery), a total of 80 rats (see: Gao E, KochWJ. A novel and efficient model of coronary artery ligation in them. Methods in Molecular Biology (Clifton, N.J. ).2013;1037:299-311):
[0025] (1) STS 20.8mg / kg / d: 20 pieces
[0026] (2) Normal saline group: 20 rats
[0027] Administration method: intraperitoneal injection of the required dose of drugs or normal saline 1 hour before ligation of the left anterior descending coronary artery; then intraperitoneal injection of corresponding doses of drugs every day.
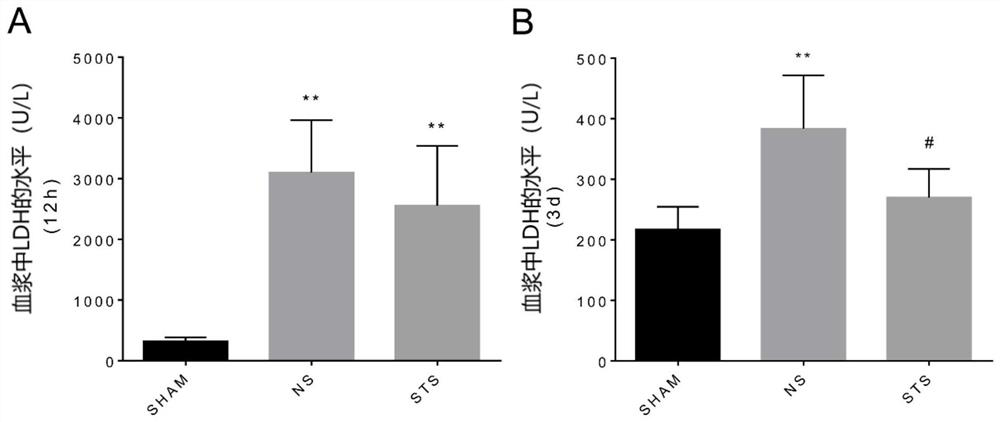
[0028] 2. Experimental results
[0029] Sodium tanshinone IIA sulfonate (STS) can reduce the level of LDH
[0030] Blood was collected from the right ventricle at 12 hours (12h) and 3 days (3d) after myocardial infarction, and the myocardial enzyme lactate dehydrogenase (LDH) was detected. Clinic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com