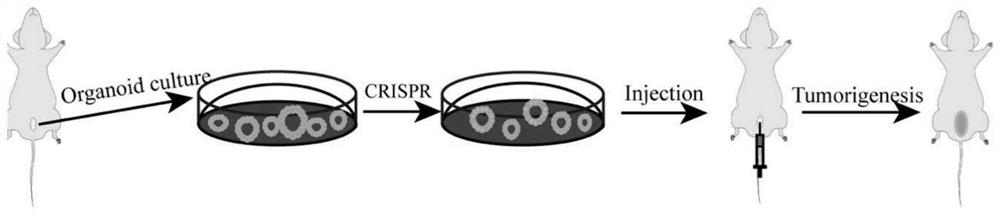
Method for constructing in-situ primary bladder cancer animal model
A bladder and animal technology, applied in the field of tumor animal models, can solve the problems of short tumor formation time, inability to be used for tumor research, and long time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1 Organoid culture of the present invention and method for expanding culture
[0069] Including obtaining fresh bladder tissue cells, trypsinization into single cells; in vitro 3D culture conditions to cultivate bladder tissue organoids.
[0070] method:
[0071] (1) Take 1-2 fresh mouse bladder tissues and cut them into pieces on ice;
[0072] (2) 8mL digestion solution (1.5mg / mL collagenase I and 1mg / mL collagenase IV+10uM Y27632) to resuspend the shredded tissue pieces (Y27632 can inhibit cell death and maintain cell activity during digestion);
[0073] (3) Digest the tissue resuspended in the digestive solution on a shaker at 37°C at a speed of 220rpm for 50min, pipetting once every 10min. Fully disperse the tissue cells;
[0074] (4) filter the digested tissue fluid with a 100 μm cell mesh;
[0075] (5) After filtration, centrifuge at room temperature, 1500rpm, and 5min to remove the supernatant;
[0076] (6) Add 3ml DMEM / F12 to resuspend, room temp...
Embodiment 2
[0090] Example 2 Gene Editing
[0091] The gene editing in this embodiment includes gene knockout and gene overexpression.
[0092] Gene knockout is: mouse bladder organoids are knocked out using CRISPR / cas9 technology. That is, the sgRNA vector and the Cas9 expression vector are packaged into a lentivirus, and then the lentivirus is transfected into an organoid, cultivated, and the gene knockout effect is verified by T7E1 enzyme. Among them, the sgRNA vector used in CRISPR / cas9 technology is pLenti-sgRNA-EFs-mcherry (with mCherry red fluorescent reporter gene).
[0093] Gene overexpression is: use the overexpression vector plasmid to perform gene overexpression in organoids, and the overexpression vector also contains a sequence for expressing luciferase. When cells are transferred into the vector plasmid, the cells can express luciferase. When the overexpression vector Bioluminescence is detected when the enzyme binds to its substrate (luciferin). The overexpression of ge...
experiment example 1
[0099] Experimental example 1 Construction of a muscle-invasive bladder cancer model
[0100] 1. Method
[0101] Using the methods in Examples 1 and 2, organoid culture, passage, and gene editing (combination A) were carried out sequentially, and the resulting gene-edited organoids were transplanted into the mouse bladder, and the size and location of the tumor were monitored by in vivo imaging technology, and transplanted for 30 Two days later, the mice were sacrificed, and bladder tissue was observed by direct observation, HE staining and immunohistochemical (IHC) staining.
[0102] 2. Results
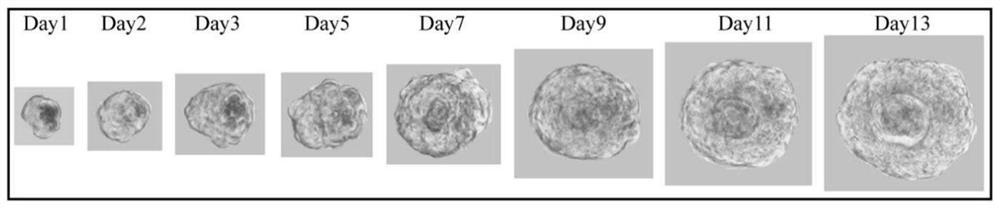
[0103] 2.1 Organoid culture
[0104] Such as figure 2 As shown, individual bladder cells gradually grow into organoids over time.
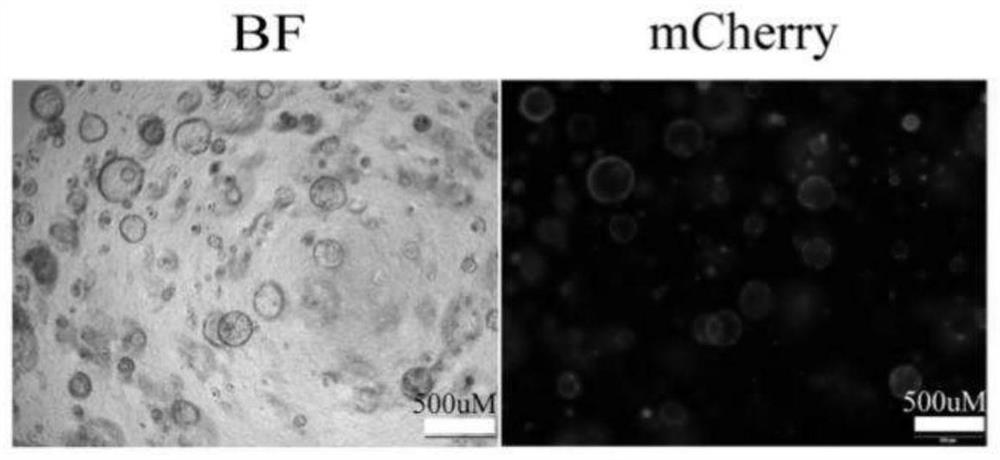
[0105] 2.2 Identification of gene editing
[0106] The results of fluorescence detection after organoid gene editing are as follows: image 3 As shown in , visible organoids have been successfully transfected with viruses for gene editing.
[010...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com