Method for constructing orthotopic primary endometrial cancer animal model
An endometrial cancer, animal model technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, animal cells, etc., can solve the problem of large differences in tumor pathophysiology, long preparation period, and can not meet the needs of model construction And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
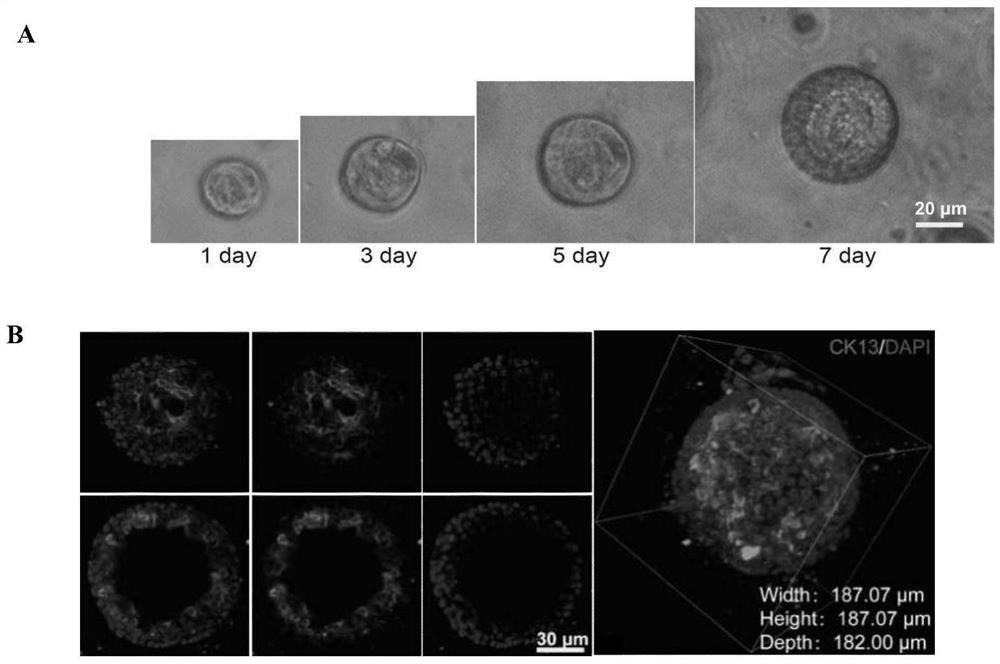
[0076] Embodiment 1 Organoid culture method of the present invention
[0077] It includes the following steps:
[0078] (1) Take fresh mouse endometrium and cut it on ice;
[0079] (2) Collagenase (2mg / mL Collagenase I and 1mg / mL Collagenase IV) to resuspend the chopped tissue blocks, and use the gentleMACS automatic tissue processor to run Mouse Tumor program 1 in the C tube; chopped tissue blocks The dosage is 1-2 grams, and the dosage of collagenase is 10 mL;
[0080] (3) The tissue block treated with collagenase was shaken at 37°C and the speed was 220rpm for 30min digestion. Make the tissue cells fully dispersed;
[0081] (4) Transfer the digested solution to the automatic tissue processor gentleMACS. On the gentleMACS, run the Mouse Tumor program 2;
[0082] (5) Filtering the liquid containing endometrial cells processed in step 4 with a 100 μm cell mesh;
[0083] (6) after filtration, at room temperature, 1500rpm, 5min centrifugation to remove supernatant;
[008...
Embodiment 2
[0093] Example 2 The modeling method of the present invention
[0094] 1. Modeling method
[0095] 1.1 Pre-culture
[0096] Same as Example 1.
[0097] 1.2 Expanding the culture
[0098] (1) Take the organoids that have been cultured for about 7 days, resuspend the digested organoids with TrypLE, transfer them to a 15mL centrifuge tube, add 3ml TrypLE to one well of a 48-well plate, and pipet 10 to 20 times until the matrigel is completely broken. , digested in a water bath at 37°C for 5 min;
[0099] (2) Take out from the water bath, pipet again for 20 to 30 times, digest at 37°C for 5 min, and then perform the third pipetting (20 to 30 times). Digestion into single cells when viewing organoids under a microscope. If a single cell is not formed, the water bath and pipetting can be repeated once until a single cell is formed.
[0100] (3) 1500rpm, centrifuge at room temperature for 5min, remove the supernatant;
[0101] (4) After counting the cells, add 30 μL of Martrig...
experiment example 1
[0135] Experimental example 1 Construction of a kind of endometrial adenocarcinoma model
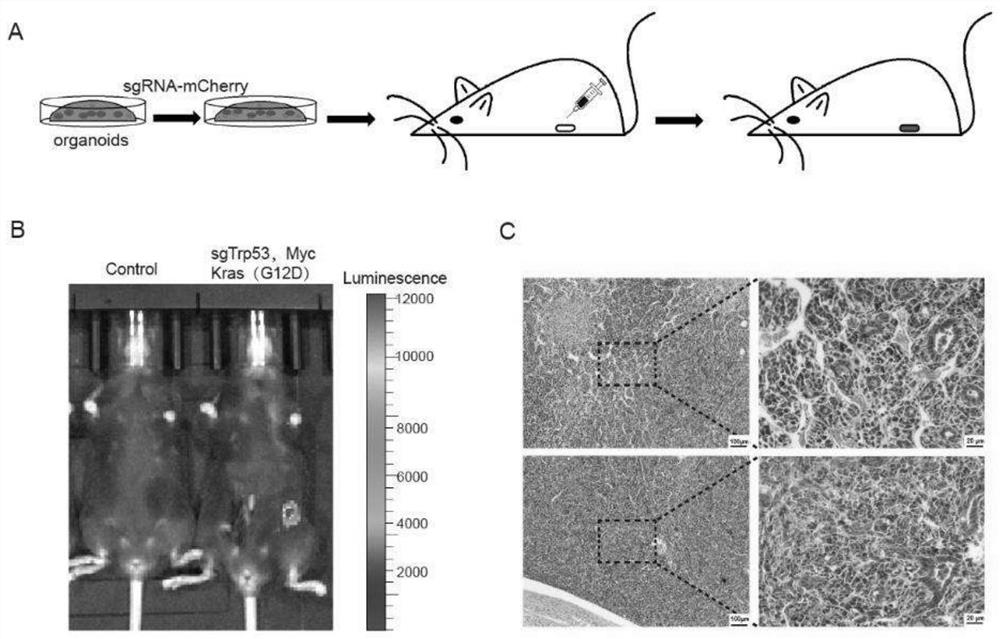
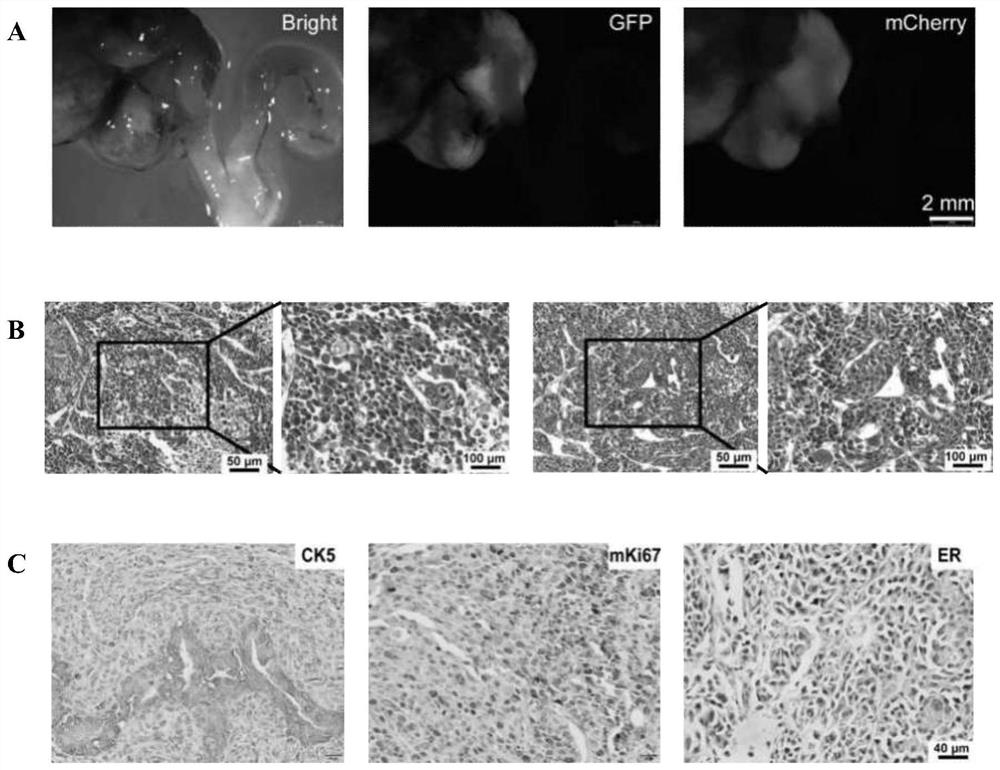
[0136] The endometrial cancer model was constructed using the method in Example 2, wherein gene combination 1 in Table 2 was selected for the gene editing part. That is, endometrial cancer organoids with knockout of Trp53 gene and overexpression of Kras(G12D) and Myc were cultured, orthotopically transplanted into the uterus of mice to construct an animal model of endometrial cancer. 135 days after transplantation, all three mice developed tumors.
[0137] The schematic diagram of the construction process is as follows figure 2 As shown in A.
[0138] figure 2 B shows the live imaging results of tumors in mice 95 days after transplantation. It can be seen that tumors formed in the uterus of mice transformed with gene-edited organoids.
[0139] figure 2 C shows that the tumor exhibits features of poorly differentiated adenocarcinoma.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com