Method for constructing in-situ primary gastric cancer animal model
An animal model and gastric cancer technology, applied in biochemical equipment and methods, animal cells, vertebrate cells, etc., can solve problems such as increased research costs, difficulty in achieving synchronization, and insufficient research needs, and achieve the effect of shortening the construction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1 Construction and verification of mouse gastric cancer model of the present invention
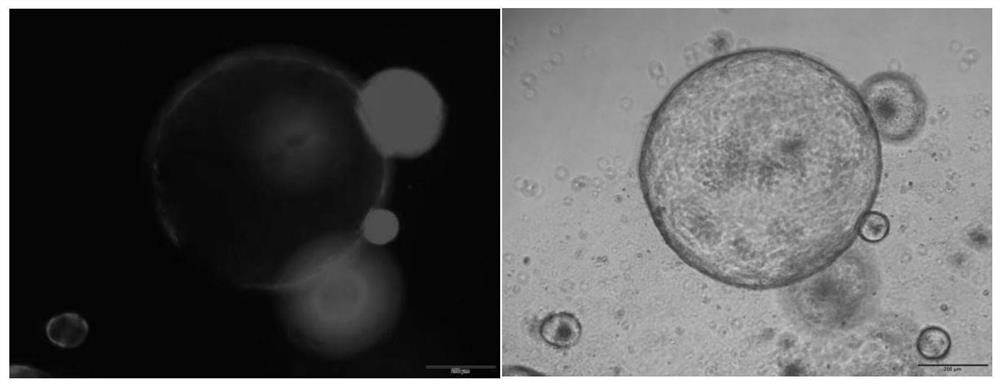
[0070] (1) Using organoid culture technology to culture gastric organoids in vitro
[0071] Take the stomach of C57BL / 6 mice, wash the gastric contents with pre-cooled DPBS solution, cut into 2-3mm size tissue pieces, pre-digest with DPBS solution containing Y27632 10μM, EDTA 5mM on ice for 30 minutes , use a 1ml pipette gun to pipet 10-20 times, observe that a large amount of gastric mucosal epithelial gland tissue is released, let it stand still, and wait for the undigested tissue pieces to settle naturally, collect the supernatant in a 15ml BD tube, centrifuge at 350g for 5min, Discard the supernatant, add 10ml of cell digestion solution TrypLE, digest at 37°C for 15min, obtain a single-cell suspension of mouse gastric mucosal epithelial cells, count them with trypan blue, and inoculate them in a 48-well plate at a density of 30,000 cells / 30ul Matrigel Put it in the i...
experiment example 1
[0091] Experimental example 1 Construction of primary and orthotopic mouse gastric cancer model with TP53 / PTEN / SMAD4 knockout
[0092] 1. Method
[0093] The gastric tissue of normal immune complete C57BL / 6 mice was taken, and the organoid culture, passage, genetic modification, and gene knockout verification were sequentially carried out using the method of Example 1, and the obtained genetically modified organoids were transplanted into the mouse stomach, and passed Live imaging technology was used to monitor the size and location of tumors. After 100 days of transplantation, mice were sacrificed, and gastric tumors were observed by direct observation, HE staining and immunohistochemical staining.
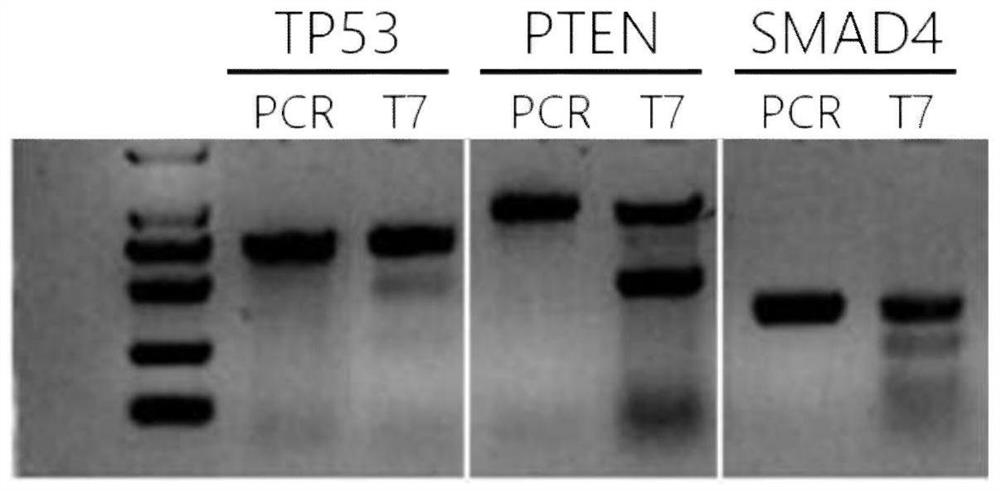
[0094] The genetic modification is to knock out tumor suppressor genes TP53, PTEN, and SMAD4 by using CRISPR / Cas9 gene knockout technology.
[0095] 2. Results
[0096] 2.1 Gene knockout verification
[0097] Detection of mutations of TP53, PTEN and SMAD4 in genetically modifi...
experiment example 2
[0105] Experimental example 2 Construction of TP53 / APC / CDKN2A / MLL3 knockout mouse gastric cancer model
[0106] 1. Method
[0107] transgenic mouse TP53 - / - , Cas9 mouse stomach (TP53 gene deletion, Cas9 protein expression) tissue, using the method of Example 1 to carry out organoid culture, passage, and genetic modification in sequence, and the resulting genetically modified organoids were transplanted into nude mouse stomach (immunodeficient small 45 days after transplantation, the mice were sacrificed, and HE staining and T7 endonuclease gene knockout verification were performed on gastric tissues.
[0108] The genetic modification is to use the CRISPR / Cas9 gene knockout technology to knock out the tumor suppressor genes APC, CDKN2A, and MLL3 (because the mice themselves are TP53 gene-deleted, TP53 will not be knocked out here).
[0109] 2. Results
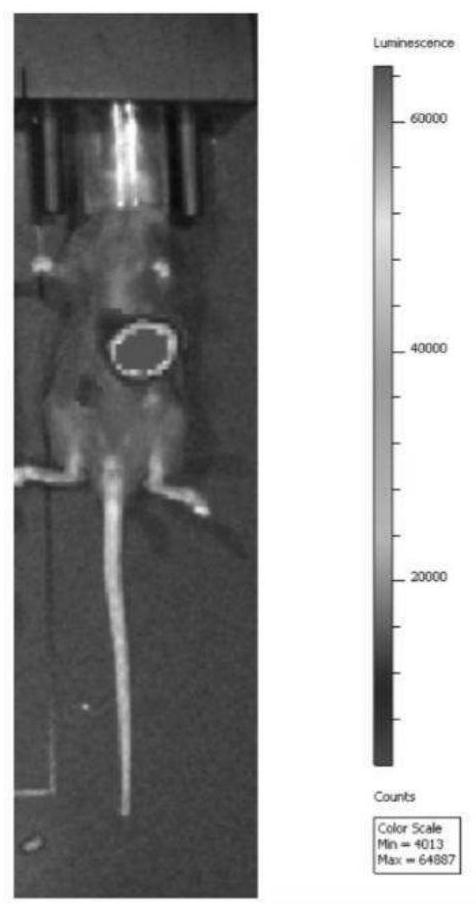
[0110] 2.1 Live tumor imaging
[0111] After 40 days of transplantation, the in vivo fluorescence imaging system was used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com