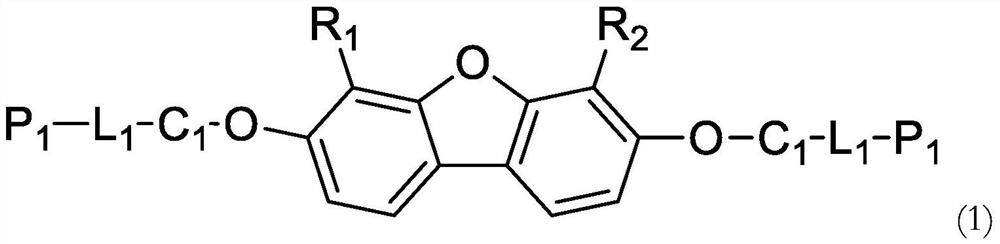
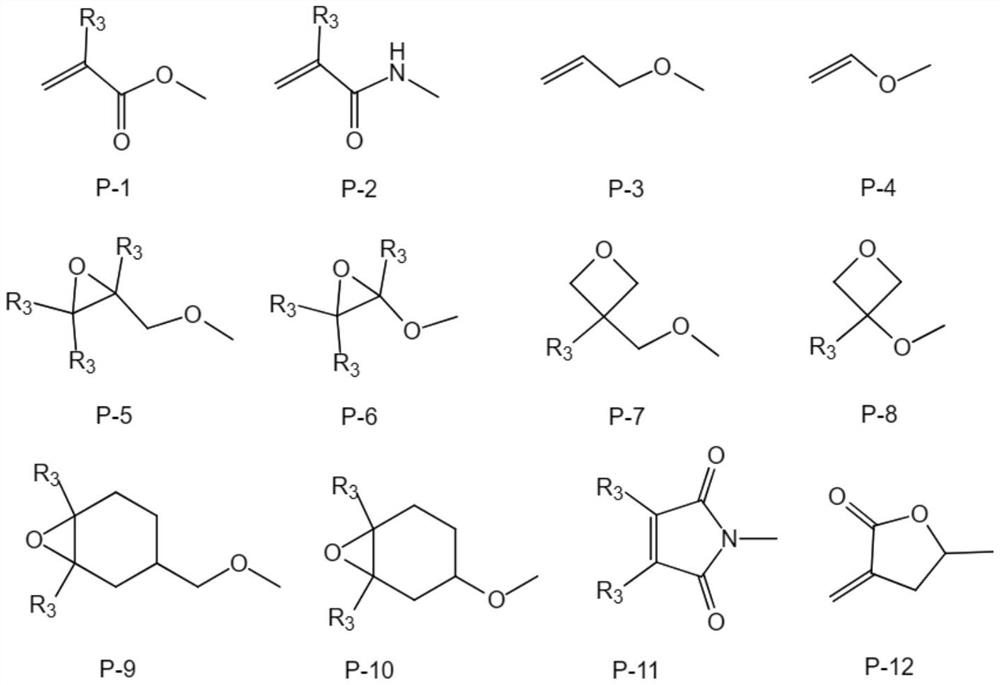
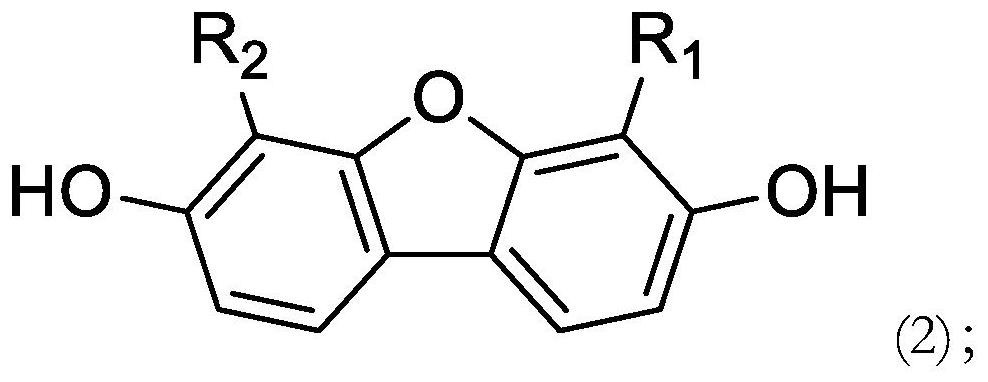
Polymerizable compound based on dibenzofuran and preparation method and application thereof
A technology of polymer compounds and polymer compositions, applied in the field of optical materials, can solve the problems of poor film non-uniformity, easy precipitation, and difficulty in stable storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0081] Synthesis of Intermediate 1:
[0082]
[0083] 4-Hydroxybutyl acrylate, dichloromethane, pyridine, dichloromethane solution with triphosgene added dropwise, keep warm for 1 hour after the dropwise addition, add water dropwise, separate liquids, collect the lower layer, concentrate to dry dichloromethane to obtain intermediate 1 for later use .
[0084]
[0085] Synthesis of Compound A:
[0086]
[0087] Add 10g of 3,7-dihydroxydibenzofuran, 31.3g of intermediate 1, and 100g of dichloromethane into the reaction flask, then add 23g of diisopropylethylamine dropwise at a controlled temperature of 0-5°C, and add In water, separated and washed once with water, dried and concentrated, crystallized in a mixed solvent of toluene and ethanol, the compound B after drying was 21.4g, content: 97.6%, yield: 78.7%. 1 H NMR (400MHz, DMSO-d 6 ): δ7.88(d,2H.),7.84(s,2H.),7.27(d,2H.),6.29(d,2H.),6.05(t,2H.),5.61(d,2H. ), 4.24(t, 4H.), , 3.98(t, 4H.), 1.63(m, 8H.).
Synthetic example 2
[0088] Synthesis Example 2: Synthesis of Compound B
[0089]
[0090] Synthesis of Compound B:
[0091]
[0092] Add 10g of 3,7-dihydroxy-4-fluorodibenzofuran, 31.3g of intermediate 1, and 100g of dichloromethane into the reaction flask, and then add 23g of diisopropylethylamine dropwise at a controlled temperature of 0-5°C. After the reaction is complete, add water, wash once with water, dry and concentrate, crystallize in a mixed solvent of toluene and ethanol, and dry compound B21.4g, content: 97.6%, yield: 78.7% 1 H NMR (400MHz, DMSO-d 6 ): δ7.88(d,1H.),7.84(s,1H.),7.63(d,1H.),7.27(d,1H.),7.25(d,1H.),6.29(d,2H. ), 6.05(t, 2H.), 5.61(d, 2H.), 4.24(t, 4H.), 3.98(t, 4H.), 1.63(m, 8H.).
Synthetic example 3
[0093] Synthesis Example 3: Synthesis of Compound C
[0094]
[0095] Add 10g of 3,7-dihydroxy-4,8-difluorodibenzofuran, 27.4g of intermediate 1 of Synthesis Example 2, and 100g of dichloromethane into the reaction flask, and then add dichloromethane dropwise at a temperature of 0-5°C. 20.5 g of isopropylethylamine was added into water after the reaction was complete, separated and washed once with water, dried and concentrated, crystallized in a mixed solvent of toluene and ethanol, and the compound C after drying was 20.7 g, content: 98.1%, yield: 82.7%. 1 H NMR (400MHz, DMSO-d 6 ):7.64(s,2H.),7.27(d,2H.),6.29(d,2H.),6.05(t,2H.),5.61(d,2H.),4.24(t,4H.), , 3.98 (t, 4H.), 1.63 (m, 8H.).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com