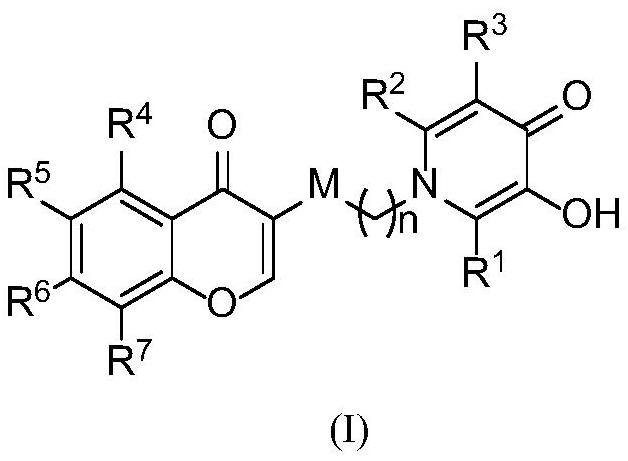
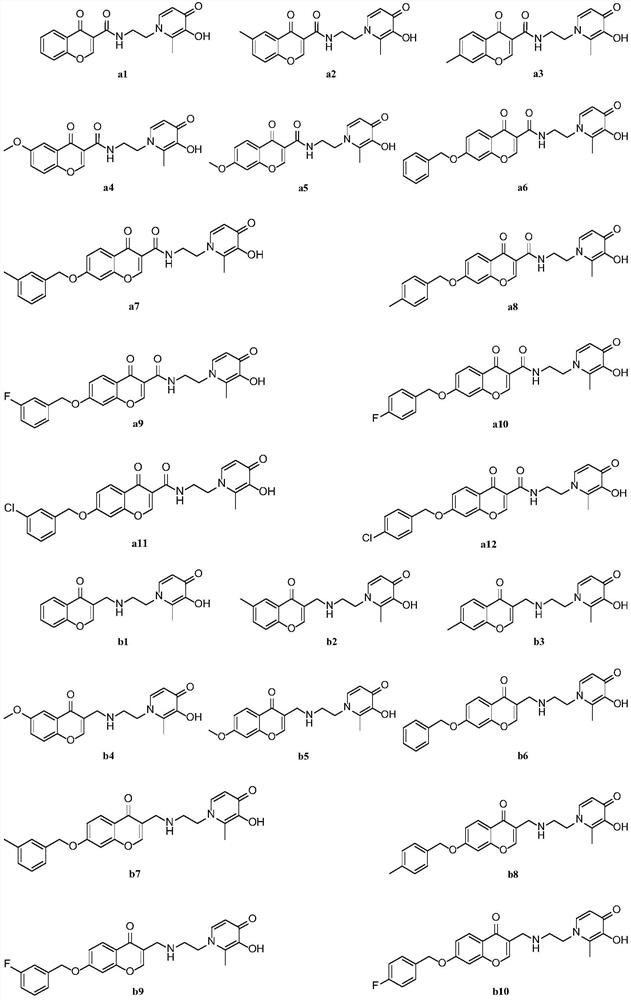
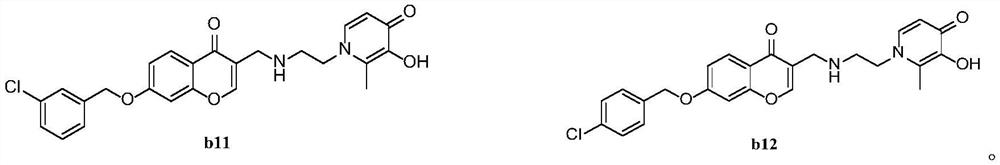
Monoamine oxidase B inhibitor with potential iron chelating activity and application thereof
A hybrid, compound technology that can be used in organic chemistry, drug combinations, neurological diseases, etc., to solve problems such as poor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] N-(2-(2-methyl-3-hydroxy-1(4H)-4-oxopyridyl)ethyl)-4-oxo-4H-chromene-3-carboxamide (a1) preparation method
[0046] Add 2-hydroxyacetophenone (1.36g, 10mmol) and anhydrous N,N-dimethylformamide (20mL) into a 100mL single-necked bottle, stir at -15°C for 1h, and then pass through a constant pressure dropping funnel Phosphorus oxychloride (3.825g, 25mmol) was slowly added dropwise to the reaction solution, and after the dropwise addition was completed, it was transferred to room temperature and continued to stir for 14 hours. After washing with diethyl ether, a light yellow solid (1.55 g) was obtained with a yield of 89%.
[0047] Add the above light yellow solid (0.522g, 3mmol) into a 250mL single-necked bottle, dissolve it in 50ml of dichloromethane, then dissolve sulfamic acid (1.748g, 18mmol) in water (40mL) and add, stir at -2°C After 0.5h, slowly add an aqueous solution (25mL) of sodium chlorite (1.628g, 18mmol) dropwise to the reaction solution through a constant...
Embodiment 2
[0052] N-(2-(2-methyl-3-hydroxy-1(4H)-4-oxopyridyl)ethyl)-4-oxo-6-methyl-4H-benzopyran-3- The preparation method of formamide (a2)
[0053] Add 2-hydroxy-5-methylacetophenone (1.50g, 10mmol) and anhydrous N,N-dimethylformamide (20mL) into a 100mL single-necked bottle, stir at -15°C for 1h, and pass Slowly drop phosphorus oxychloride (3.825g, 25mmol) into the reaction solution with a constant pressure dropping funnel, transfer to room temperature after the dropwise addition and continue to stir for 14h. After the reaction, pour into ice water to precipitate solids, filter, After washing with water and then with ether, a light yellow solid (1.64 g) was obtained with a yield of 87.2%.
[0054] Add the above light yellow solid (0.564g, 3mmol) into a 250mL single-necked bottle, dissolve it in 51ml of dichloromethane, then dissolve sulfamic acid (1.748g, 18mmol) in water (42mL) and add, stir at -2°C After 0.5h, slowly add an aqueous solution (25mL) of sodium chlorite (1.628g, 18mm...
Embodiment 3
[0059] N-(2-(2-methyl-3-hydroxy-1(4H)-4-oxopyridyl)ethyl)-4-oxo-7-methyl-4H-benzopyran-3- The preparation method of formamide (a3)
[0060] Add 2-hydroxy-4-methylacetophenone (1.50g, 10mmol) and anhydrous N,N-dimethylformamide (20mL) into a 100mL single-necked bottle, stir at -15°C for 1h, and pass Slowly drop phosphorus oxychloride (3.825g, 25mmol) into the reaction solution with a constant pressure dropping funnel, transfer to room temperature after the dropwise addition and continue to stir for 14h. After the reaction, pour into ice water to precipitate solids, filter, Washed with water and then with diethyl ether to obtain a light yellow solid (1.59 g), yield 84.6%.
[0061] Add the above light yellow solid (0.564g, 3mmol) into a 250mL single-necked bottle, dissolve it in 51ml of dichloromethane, then dissolve sulfamic acid (1.748g, 18mmol) in water (42mL) and add, stir at -2°C After 0.5h, slowly add an aqueous solution (25mL) of sodium chlorite (1.628g, 18mmol) dropwise...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com