Expression method of efficient molecular three-dimensional conformation
An expression method and three-dimensional structure technology, applied in the field of computer-aided drug research and development, can solve the problems of data reading and writing IO bottlenecks, large file storage space, and occupying huge file storage space, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A method for expressing a high-efficiency molecular three-dimensional conformation of this embodiment includes the following steps:
[0045] Step A, establishing an auxiliary file of the basic fragment library to obtain the three-dimensional structure data of the basic fragments that need to be referenced in all three-dimensional conformation metadata files; the basic fragment library includes the following information:
[0046] (1) The unique representation code of the basic fragment is the hash code;
[0047] The basic fragment is the smallest fragment unit combined into a molecule, and the unique characterization code of the basic fragment is a hash code, which is completely uniquely determined by the topology and stereo information (such as molecular chirality and stereo relationship) of the basic fragment molecule. Decide;
[0048] (2) Whether the basic fragment is in the public library or customized;
[0049] (3) If the basic fragment is self-defined, how many t...
Embodiment 2
[0075] The specific implementation manner of this embodiment uses CH 3 -SiH 2 -GeH 2 -OH molecule as an example, the expression method of the three-dimensional conformation of the molecule is as follows:
[0076] 1. Establish the auxiliary file of the basic fragment library to obtain the three-dimensional structure data of the basic fragments that need to be referenced in all three-dimensional conformation metadata files:
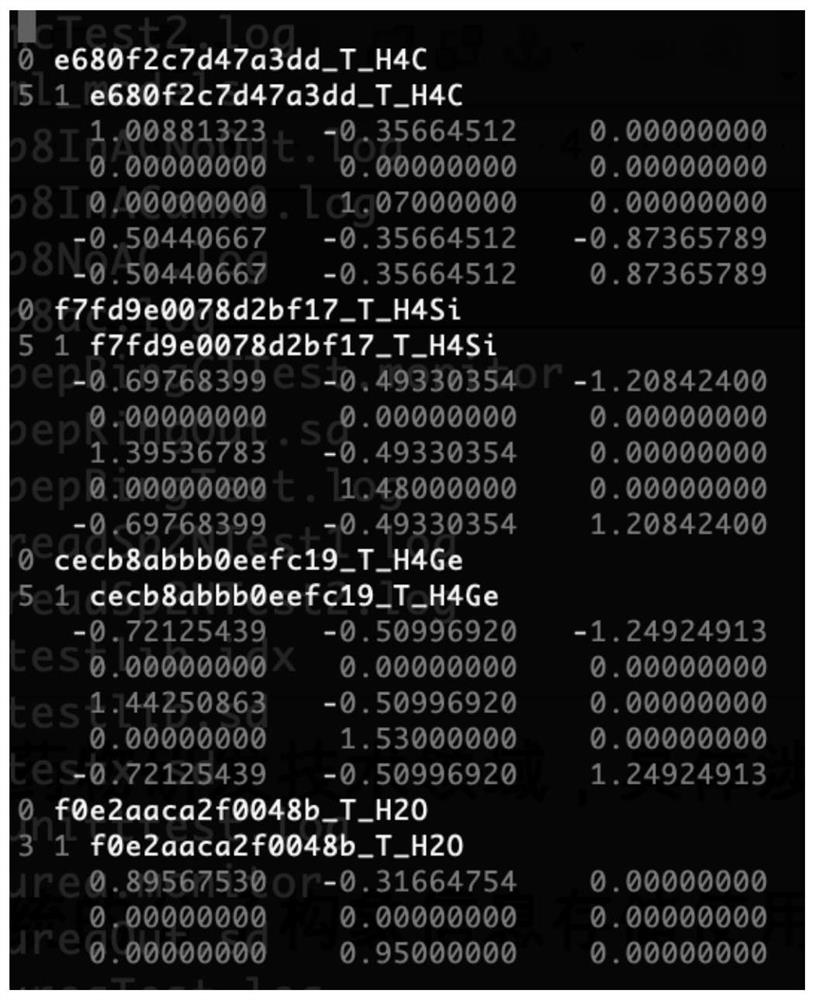
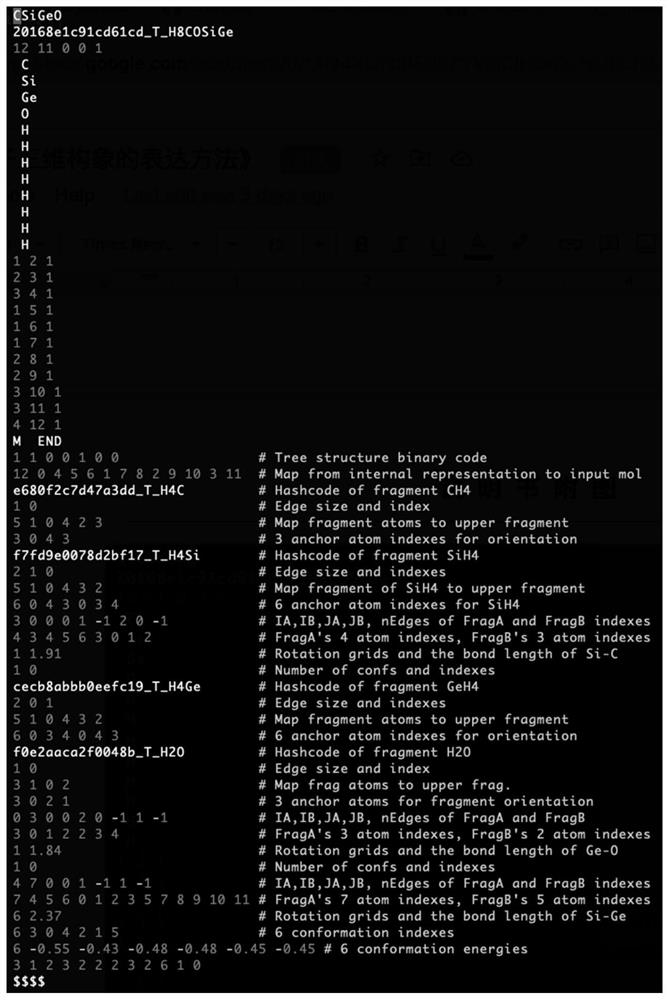
[0077] The base snippet library helper files such as figure 1 Shown:
[0078] First line: The filename of the base fragment library. This line is empty, indicating that all base fragments are custom.
[0079] Second row: Record the expression of each underlying fragment starting from the second row. The first line of each record indicates whether the record is custom (0) or public library (1) and the hash code of the record's underlying fragment.
[0080] If the base fragment is custom (0), then the line immediately above is how many atoms and how ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com