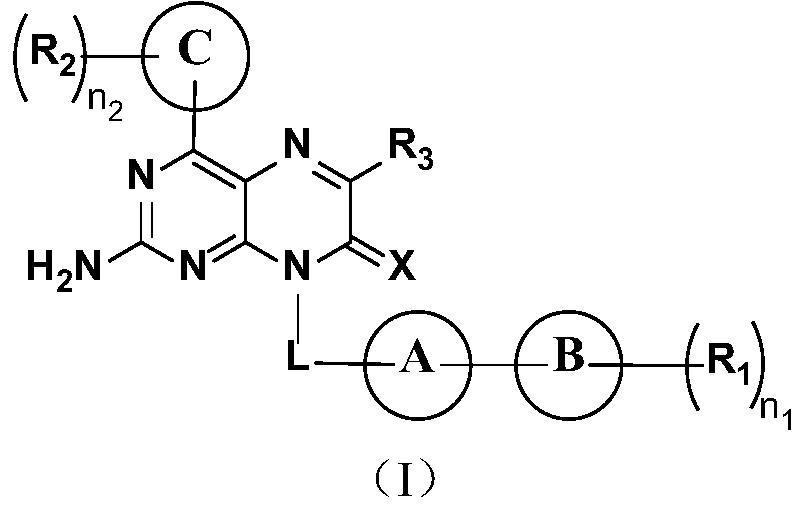
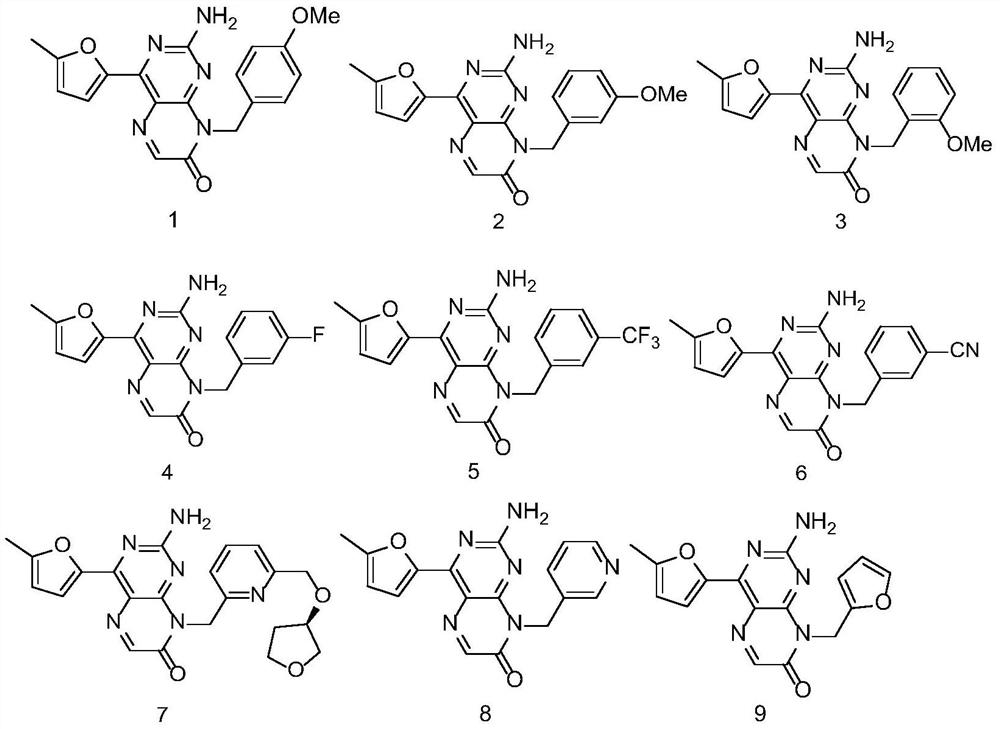
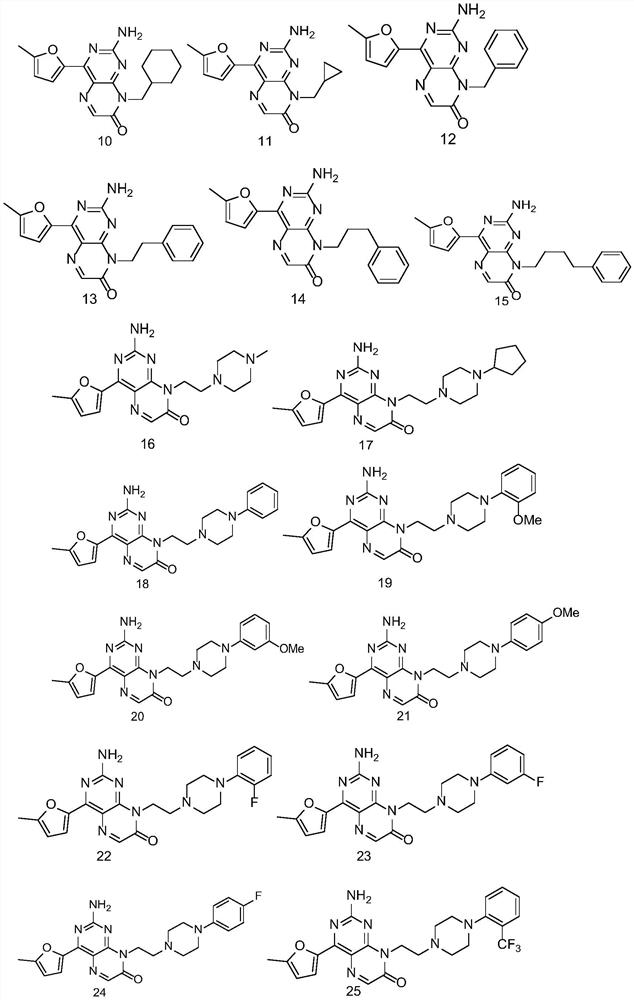
2-amino pteridinone derivative or salt thereof as well as preparation method and application of 2-amino pteridinone derivative or salt thereof
An alkyl and aryl technology, applied in the field of 2-aminopteridone derivatives or their salts and their preparation, can solve the problems of low response rate of immunotherapy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] Embodiment one 2-Amino-8-(4-methoxybenzyl)-4-(5-methylfuran-2-yl)pteridin-7(8H)-one
[0162] (2-Amino-8-(4-methoxybenzyl)-4-(5-methylfuran-2-yl)pteridin-7(8H)-one)
[0163]
[0164] Step 1: 6-Chloro-N 4 Synthesis of -(4-methoxybenzyl)pyridine-2,4,5-triamine
[0165] 2,5-diamino-4,6-dichloropyrimidine (1.79g, 10.0mmol, 1.0equiv.), 4-methoxybenzylamine (2.06g, 15.0mmol, 1.5equiv.), Et 3 N (2.02 g, 20.0 mmol, 2.0 equiv.) was dissolved in an appropriate amount of Sec-BuOH, and stirred at 105° C. for 12 hours under nitrogen protection. TLC monitored the reaction in real time. After completion of the reaction, stand at 2-8°C until the solid precipitates, filter, wash with cold ethanol and petroleum ether successively, and dry to obtain a pink solid (2.0 g, yield: 71.5%).
[0166] ESI(m / z):[M+1] + 279.9; 1 H NMR (400MHz, DMSO-d 6 )δ7.25(d, J=8.4Hz, 2H), 6.94(s, 1H), 6.87(d, J=8.5Hz, 2H), 5.67(s, 2H), 4.47(s, 2H), 3.93( s,2H),3.71(s,3H).
[0167] Step 2: Synthesis ...
Embodiment 2
[0173] Embodiment two 2-Amino-8-(3-methoxybenzyl)-4-(5-methylfuran-2-yl)pteridin-7(8H)-one
[0174] (2-Amino-8-(3-methoxybenzyl)-4-(5-methylfuran-2-yl)pteridin-7(8H)-one)
[0175]
[0176] Route with reference to embodiment 1.
[0177] ESI(m / z):[M+1] + 363.8; 1 H NMR (400MHz, DMSO-d 6 )δ7.90(s,1H),7.77(d,J=3.5Hz,1H),7.52(brs,2H),7.21(t,J=7.9Hz,1H),6.97(s,1H),6.92( d,J=7.5Hz,1H),6.82(d,J=8.1Hz,1H),6.39(d,J=3.6Hz,1H),5.34(s,2H),3.72(s,3H),2.41( s,3H).
Embodiment 3
[0178] Embodiment three 2-Amino-8-(2-methoxybenzyl)-4-(5-methylfuran-2-yl)pteridin-7(8H)-one
[0179] (2-Amino-8-(2-methoxybenzyl)-4-(5-methylfuran-2-yl)pteridin-7(8H)-one)
[0180]
[0181] Route with reference to embodiment 1.
[0182] ESI(m / z):[M+1] + 363.8; 1 H NMR (400MHz, DMSO-d 6 )δ7.93(s,1H),7.81(d,J=3.4Hz,1H),7.56(brs,2H),7.21(d,J=8.0Hz,1H),7.02(d,J=8.2Hz, 1H), 6.78(t, J=7.3Hz, 1H), 6.58(d, J=7.6Hz, 1H), 6.41(s, 1H), 5.31(s, 2H), 3.87(s, 3H), 2.41( s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com