Biomarkers and methods of diagnosis and treatment for recurrent implant failure
A biomarker and biological technology, applied in the field of in vitro fertilization-embryo implantation, can solve the limitations and changes of RIF diagnosis and treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0248] In order to explore the relationship between luteinizing hormone and RIF, the periodic changes of serum levels of luteinizing hormone LH in RIF patients were detected, and compared with the periodic changes of LH levels in other IVF patients.
[0249] In the previous study, patients with secondary infertility caused by tubal factors alone, aged 25-36 years old (average 27.5±2.4), without a history of embryonic cessation, were selected, and no hormone drugs were used within three months. The LH value on the 3rd and 5th day after ovulation. The average LH level of the patient population on D3 was 7.63±3.49mIU / ml; the average LH level on D5 was 6.67±4.53mIU / ml, and did not show low LH levels in the luteal phase. In this embodiment, patients who underwent IVF-ET due to tubal factors alone were selected as controls.
[0250] In short, in this example, patients with a history of RIF were selected as the test group; at the same time, patients who underwent IVF-ET due to tubal...
Embodiment 2
[0261] For recurrent implantation failures with low LH serum levels in the luteal phase, a new luteal support regimen supplemented with the LH functional analogue HCG was given, and the relevant clinical treatment responsiveness of the patients was retrospectively observed. In this retrospective observational study, according to the previous dynamic observation of LH levels in RIF patients and tubal factor IVF patients, the serum LH level on D2 day of the luteal phase was ≤5 IU / L as one of the enrollment conditions.
[0262] All patients enrolled in this study were from the Sixth Medical Center of the General Hospital of the Chinese People's Liberation Army; and signed informed consent. All clinical studies were approved by the institutional ethics committee before being conducted.
[0263] A total of 84 patients were enrolled. The general health status of the patient is good, and there is no history of immunization, chromosomal abnormalities, and recurrent miscarriage; ,35(...
Embodiment 3
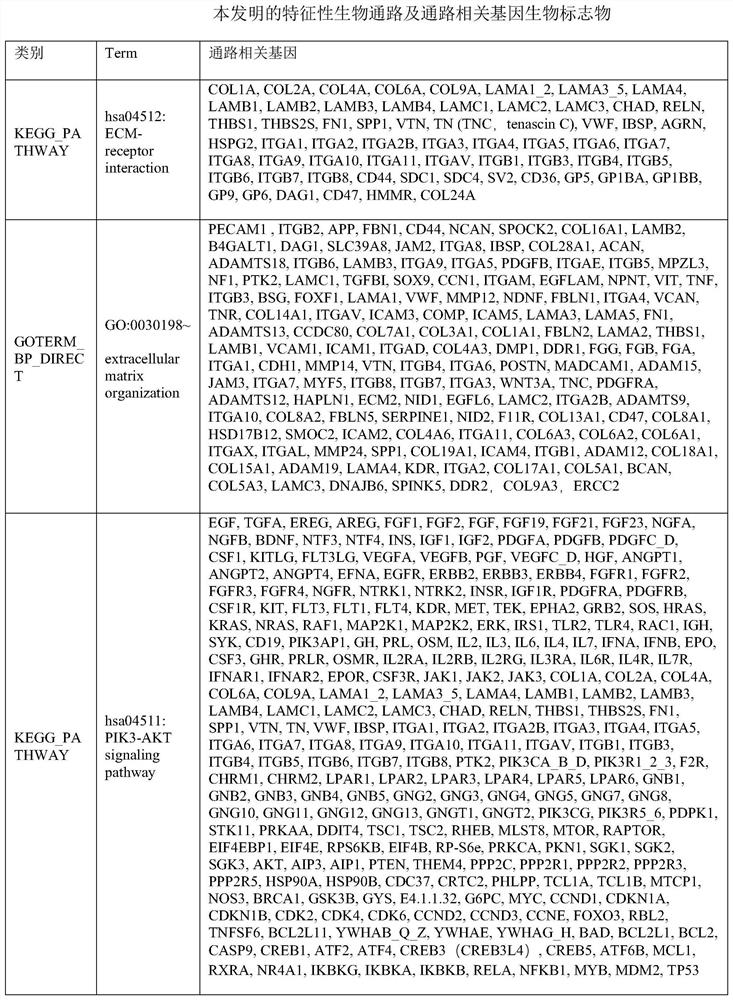
[0287] To examine the genetic basis of responsiveness to HCG luteal support therapy in RIF patients by whole-genome exome sequencing and functional enrichment analysis.
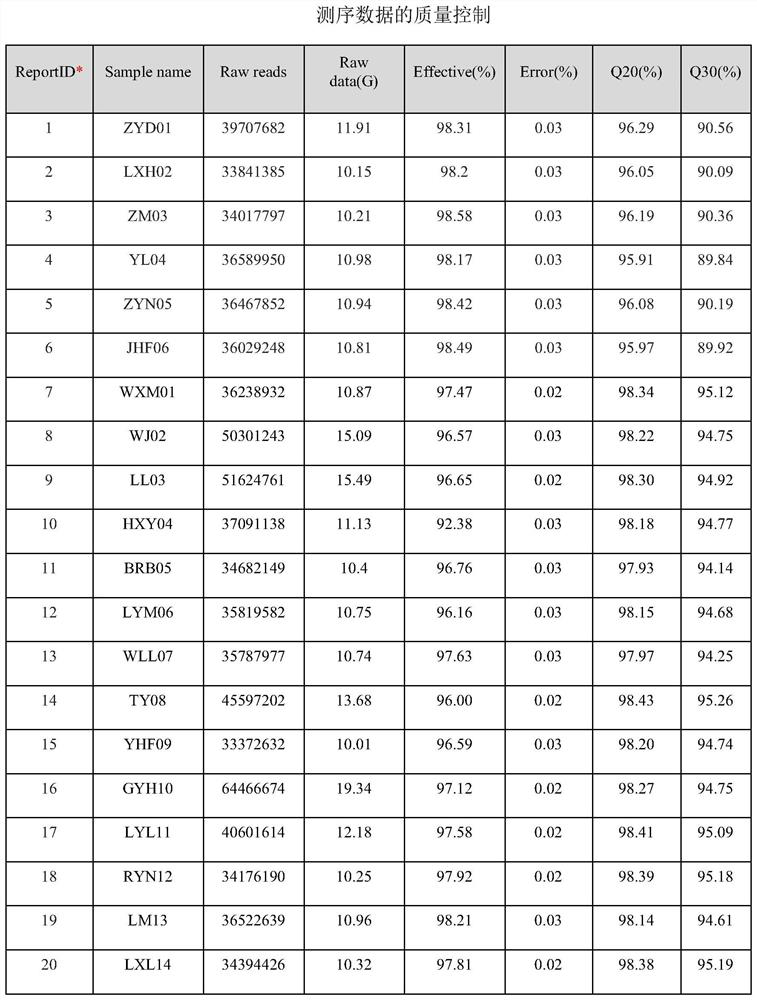
[0288] In this study, 10 patients were randomly selected from the RIF patients who achieved successful clinical pregnancy by using the HCG supplementary luteal phase support treatment regimen described in Example 2 as the case group. At the same time, 10 patients with RIF who had not been tested for LH hormone levels and received luteal support with HCG were randomly selected as the control group. All patients included in the study were from the Sixth Medical Center of the General Hospital of the Chinese People's Liberation Army; and signed informed consent. A comparison of patient characteristics between case and control groups is shown in Table 5.
[0289] Table 5 Comparison of general demographic characteristics of patients between the two groups
[0290]
[0291] Note: P<0.05 was regarded as statisti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com