Methods for detecting adverse local tissue reaction (ALTR) necrosis
A local tissue, poor technology, used in biochemical equipment and methods, measuring devices, pharmaceutical formulations, etc., can solve problems such as infection, difficult to explain scanning, low incidence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0324] Example 1: Confirmatory necrosis analysis to determine the need for revision surgery in MOM total hip arthroplasty
[0325] A study was designed to develop a biomarker assay for use as a diagnostic tool for ALTR in subjects with total hip replacement (THR) with MOM.
[0326] To identify biomarkers of ALTR, the composition of samples from ALTR patients needs to be compared with samples from patients of different disease categories. Molecules (typically proteins) present in ALTR patients but absent in other disease groups or normal individuals are potential biomarkers of ALTR. A critical aspect of a biomarker discovery program is the acquisition and testing of well-characterized patient samples from multiple disease categories.
[0327] This study is a multicenter prospective cohort study. To maximize the chances of successful identification of serum biomarkers, all samples were analyzed using a multi-analyte assay biomarker test. The primary endpoint of the study in...
Embodiment 2
[0329] Example 2: Target Biomarkers
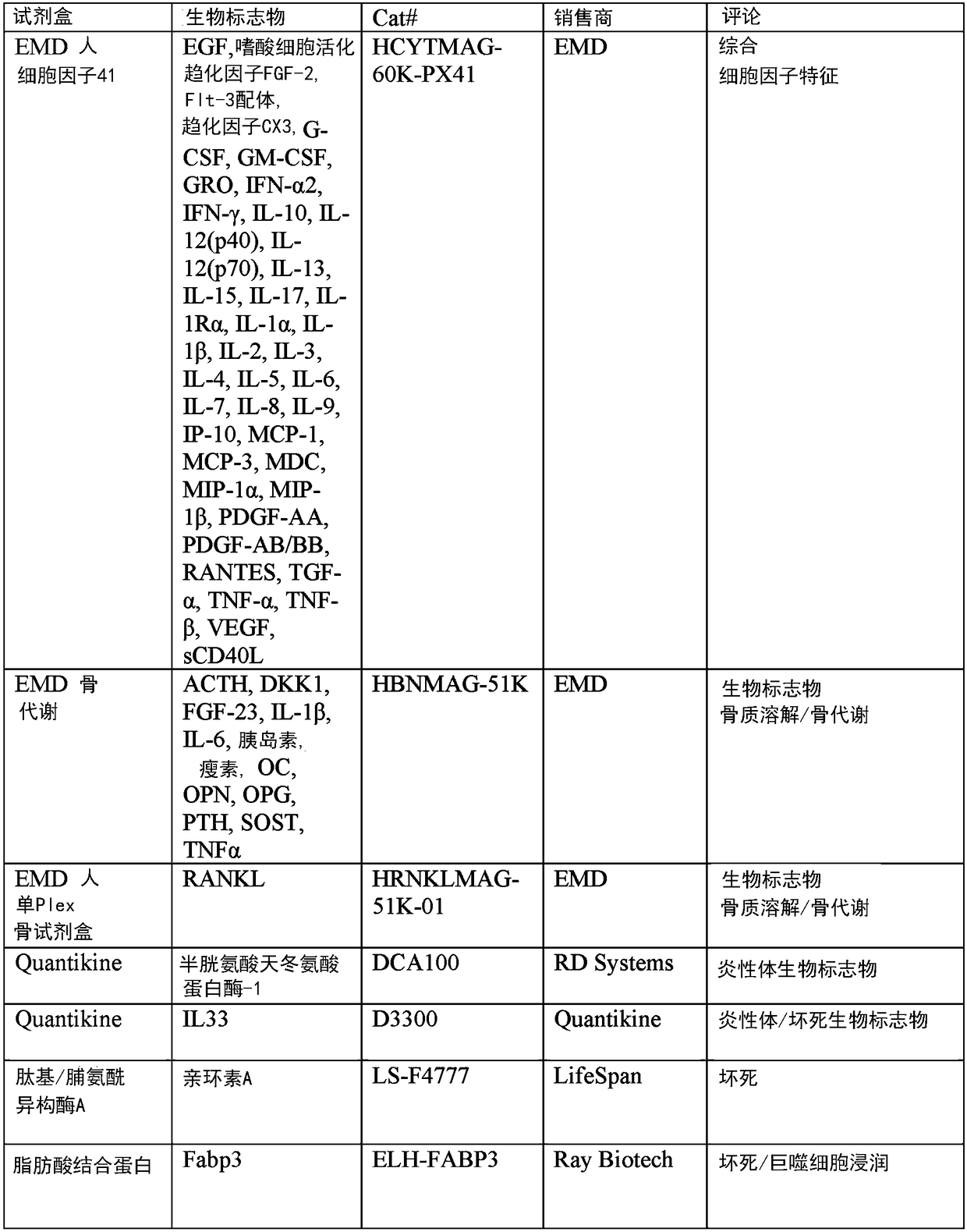
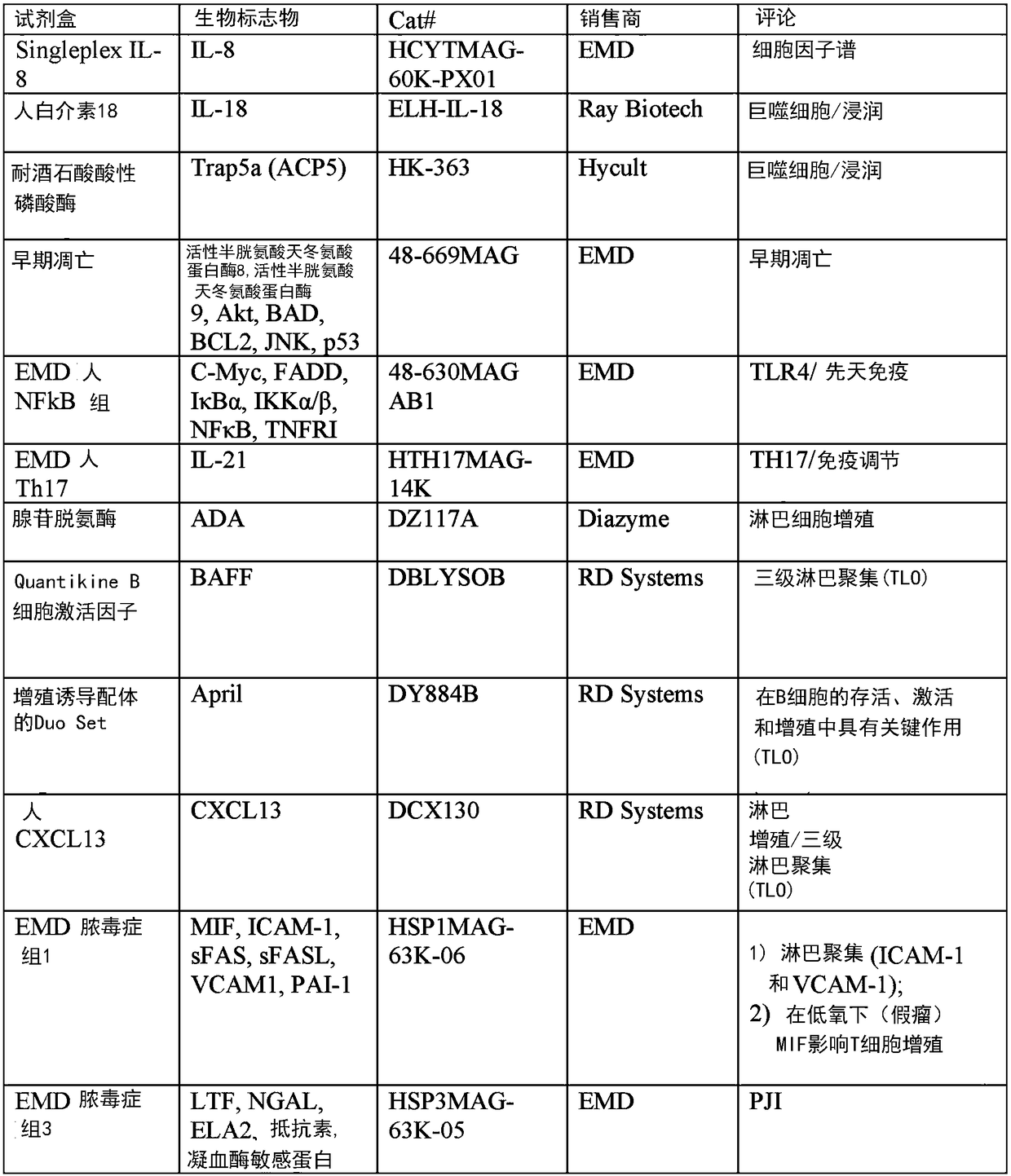
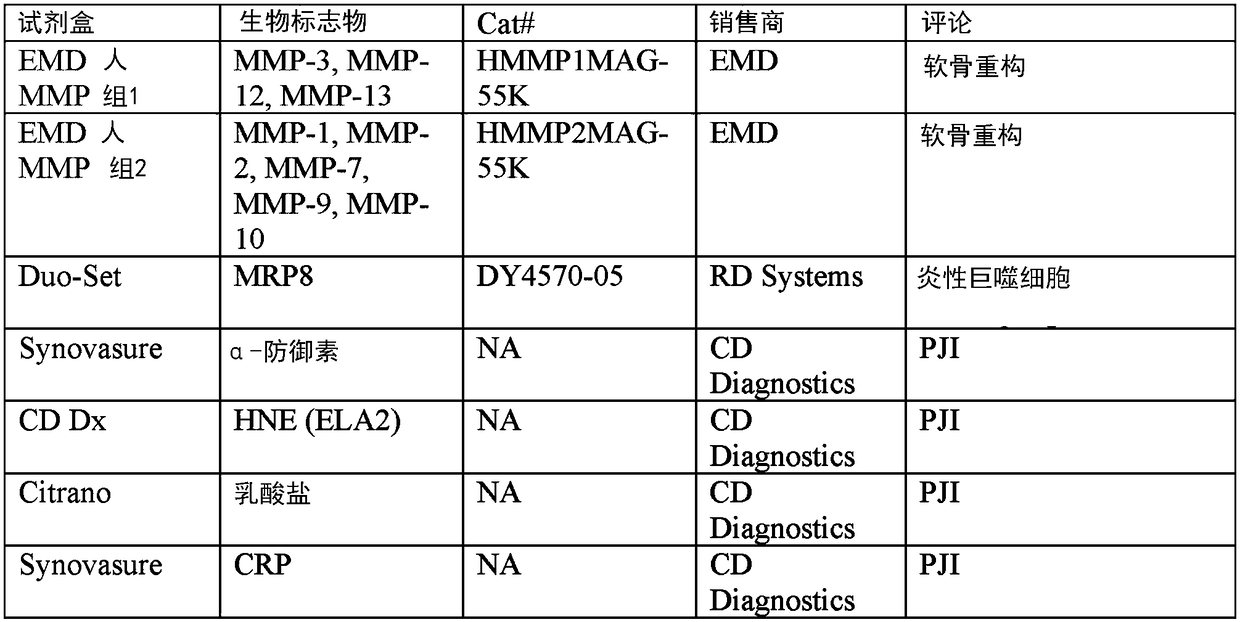
[0330] Such biomarker proteins were selected for analysis if their molecular mechanisms involved in the observed histopathology of ALTR were described or suspected. Specifically, macrophages, lymphocytes, T cell-mediated immunity, delayed-type hypersensitivity, innate immunity, necrosis, apoptosis, cell proliferation, osteolysis, wound healing, bone remodeling, and oxidative stress were selected. Biomarkers. Biomarkers of general inflammation and PJI were also included. In total, 8 multiplex Luminex assays were used to analyze 82 different biomarkers. Additionally, 17 biomarkers were analyzed using single ELISA and enzyme assays (Figure 1).
Embodiment 3
[0331] Example 3: Preliminary screening of biomarkers
[0332] With a large number of target biomarkers (99), the process of identifying ALTR biomarkers begins with assembling a relatively small number of synovial fluid samples to be analyzed. All assays were performed according to the manufacturer's recommendations with minor modifications. In addition to MOM samples, pooled synovial fluid samples from patients with PJI, OA, and sterile joints were used as controls ( figure 2 ).
[0333] Synovial fluid was prepared from patients with aseptic joints (3 sterile samples pooled together), osteoarthritis (OA) (5 OA samples pooled together), and PJI (4 PJI samples pooled together) at the initial screening Samples were pooled and 5 individual MOM samples were analyzed in the assay for 99 human biomarkers. As used herein, sterile samples were taken from subjects with low probability of ALTR or PJI; OA samples were taken from OA subjects who did not undergo joint replacement sur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com