Novel peptides
A kind of peptide compound, the technology of compound, be applied in the purposes in the treatment such as inflammation, comprise the field of pharmaceutical composition of described peptide, can solve the problems such as the progress of unfavorable wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0265] Ala-Lys-Pro-Ser-Tyr-Hyp-Thr-Tyr-Hyp-Lys (SEQ ID No: 15)
[0266] Fmoc-Lys-Boc-Wang resin (9.15 g, GLS180322-41301, GL Biochem, Shanghai, China) was loaded into a glass reaction column.
[0267] Dichloromethane (DCM, 200 mL; Shandong Jinling Chemical Industry Co Ltd, Shandong, China) was added to the column and the resin was allowed to soak for about half an hour. DCM was then removed by vacuum filtration.
[0268] The resin was washed 3 times with N,N-dimethylformamide (DMF, 200 mL; Shandong Shitaifeng Fertilizer Industry Co Ltd, Shandong, China).
[0269] A 20% piperidine solution (200 mL) in DMF was added as a deprotection solution and reacted for 20 minutes. The solution was then removed by vacuum filtration and the column was washed six times with DMF. Fmoc-4-Hyp(tBu)-OH (GLS 21303; GLBiochem, Shanghai, China) and 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyltetrafluoroboric acid Ammonium (TBTU, 2.89 g; GLS 170805-00705, GL Biochem, Shanghai, China) was added t...
Embodiment 2
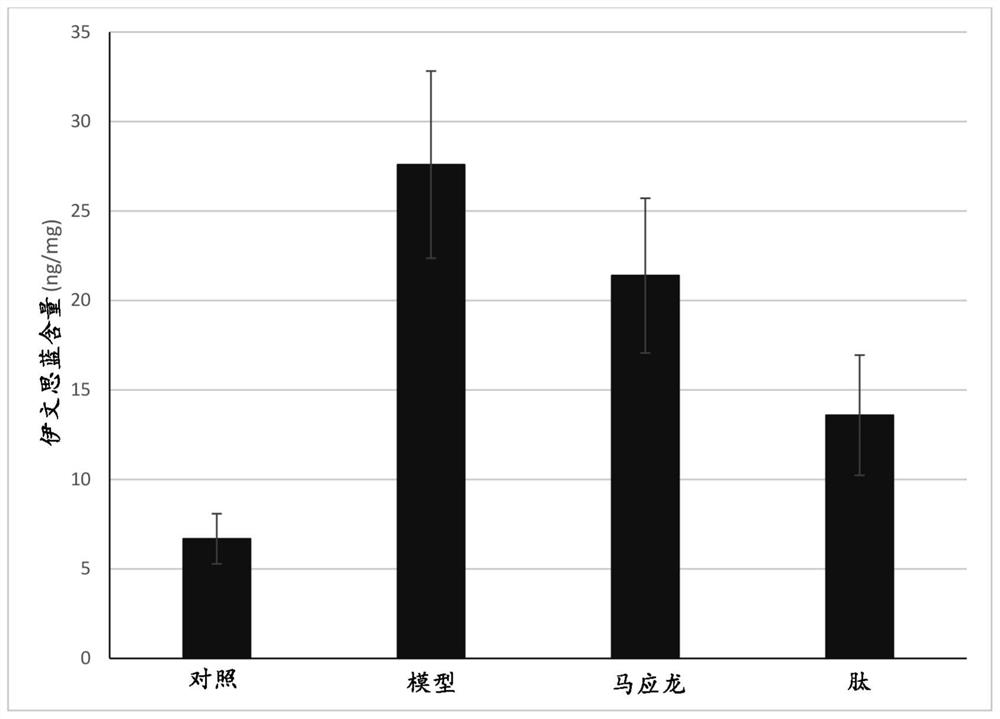
[0283] Croton oil-induced rat anal swelling model
[0284] A gel was prepared containing 0.5 g of the peptide Ala-Lys-Pro-Ser-Tyr-Hyp-Thr-Tyr-Hyp-Lys (SEQ ID No: 15; see Example 1 above), which was also prepared from Component composition: methylcellulose (2.2g; ShandongGuangda Technology Development Co., Ltd., Shandong, China), glycerin (11g) and propylene glycol 11g (both from Sinopharm Chemical Reagent Co.Ltd.) and purified water (75.3g) .
[0285] The methylcellulose and water were mixed together and stirred until a homogeneous colloidal suspension was formed. Then, the peptide powder, glycerin and propylene glycol were added to the methylcellulose / water mixture, and the resulting mixture was stirred rapidly for 5 minutes to obtain the finished product.
[0286] Six- to eight-week-old SD rats with an average weight between 180 and 220 g were provided by Changzhou Cvens Experimental Animal Co. Ltd. (Changzhou, Jiangsu Province, China), half male and half male. Rats wer...
Embodiment 3
[0301] Ala-Lys-Pro-Ser-Tyr-Hyp-Thr-DOPA-Hyp-Lys (SEQ ID No: 14)
[0302] The title compound was prepared using essentially the same method as the latter described in Example 1 above, except that Fmoc-DOPA(acetonide)-OH was used in place of Fmoc-Tyr(tBu in the relevant amino acid coupling step )-OH, yielding 3.73 g of crude title compound.
[0303] Analysis showed that the peak of interest eluted at 9.297 minutes with the expected molecular weight (MS: m / z 1199.1). The purity is 74.493%.
[0304] 3.7 g of the crude product were then purified as described above in Example 1 to give 2.01 g of pure title compound after lyophilization.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com