Synthesis method of 6-methyl nicotine
A technology of methyl nicotine and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of difficult separation of 6-methyl nicotine, unfixed methyl substitution position, harsh reaction conditions, etc., so as to avoid difficult separation, poor selectivity, conditionally controlled effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
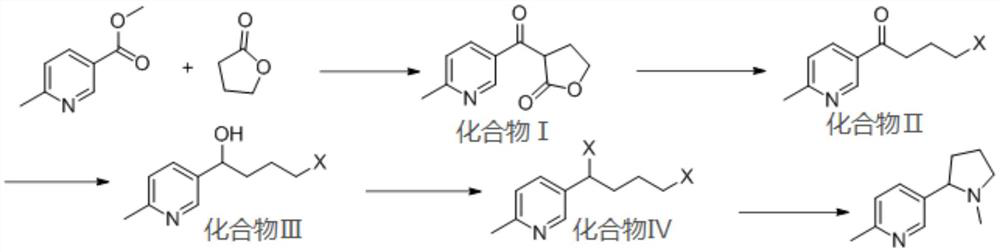
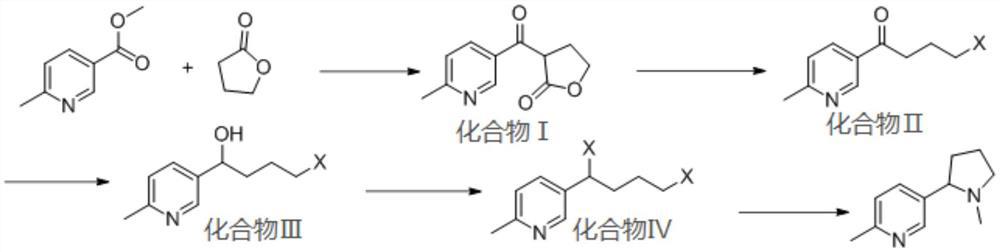
[0051] The synthesis route of 6-methylnicotine of the present embodiment is as follows:
[0052] The specific operation steps are as follows:
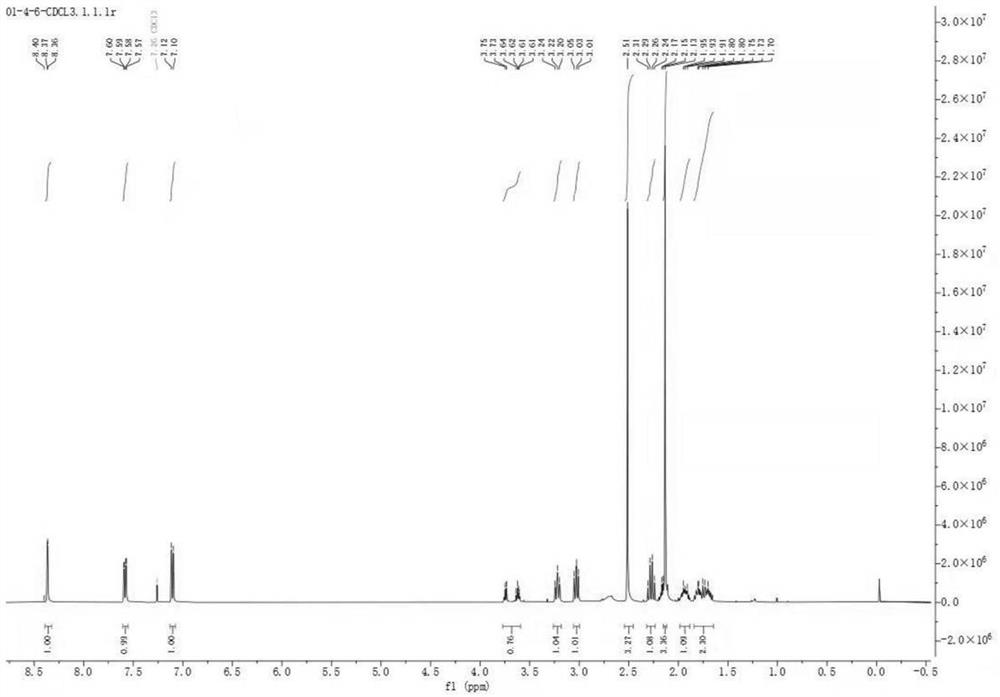
[0053] S1. Weigh 800mg of γ-butyrolactone (9.3mmol) and dissolve it in 150ml of N,N-dimethylformamide (DMF), cool down to 0°C, stir for 10min, then add 240mg of NaH (9.9mmol) in batches, and react After 30 min, 1 g of methyl 6-methylnicotinate (6.6 mmol) was added, the refrigeration was turned off, and the reaction was carried out at room temperature for 5 h, and the end point was determined by TLC detection to obtain compound I;
[0054] S2. First add a small amount of 5w% dilute hydrochloric acid to compound I until no bubbles are generated, then add 20ml concentrated hydrochloric acid and 20ml 1,4-dioxane, heat to 95°C and react for 5h, after the complete reaction of compound I is determined by TLC detection, Cool down to room temperature, add 50% NaOH solution under ice bath conditions to adjust the pH to 9, combine the organic ph...
Embodiment 2
[0060] S1. Weigh 800mg of γ-butyrolactone (9.3mmol) and dissolve it in 150ml of tetrahydrofuran, cool down to 0°C, stir for 10min, then add 240mg of NaH (9.9mmol) in batches, react for 30min, then add 1g of 6-methylnicotinic acid Methyl ester (6.6mmol), turn off the refrigeration, react at room temperature for 5h, and determine the end point by TLC detection to obtain compound I;
[0061] S2. First add a small amount of 5w% dilute hydrochloric acid to compound I until no bubbles are generated, then add 20ml concentrated hydrochloric acid and 20ml 1,4-dioxane, heat to 95°C and react for 5h, after the complete reaction of compound I is determined by TLC detection, Cool down to room temperature, add 50% NaOH solution under ice bath conditions to adjust the pH to 9, combine the organic phases after extraction and concentrate and dry to obtain compound II;
[0062] S3. Compound II was dissolved in 20ml of methanol, 250mg of sodium borohydride was added, and reacted at -10°C for 2 h...
Embodiment 3
[0066] S1. Weigh 800mg of γ-butyrolactone (9.3mmol) and dissolve it in 150ml of tetrahydrofuran, cool down to 0°C, stir for 10min, then add 960mg of sodium tert-butoxide (9.9mmol) in batches, react for 30min, then add 1g of 6-methanol Nicotinic acid methyl ester (6.6 mmol), turn off the refrigeration, react at room temperature for 5h, and determine the end point by TLC detection to obtain compound Ⅰ;
[0067] S2. First add a small amount of 5w% dilute hydrochloric acid to compound I until no bubbles are generated, then add 20ml concentrated hydrochloric acid and 20ml 1,4-dioxane, heat to 95°C and react for 5h, after the complete reaction of compound I is determined by TLC detection, Cool down to room temperature, add 50% NaOH solution under ice bath conditions to adjust the pH to 9, combine the organic phases after extraction and concentrate and dry to obtain compound II;
[0068] S3. Compound II was dissolved in 20ml of methanol, 250mg of sodium borohydride was added, and rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com